Improving the Diagnosis of Bacterial Rejections in Ovine Abattoirs by the Use of Simple Protocols
Parasitology Group, Animal Reproduction Dep. (INIA) 28130-Valdeolmos (Madrid, Spain).
*Corresponding author:
Felix Valcarcel;Email:
valcarcel.felix@inia.esViews 2931
Abstract
Veterinary inspection in abattoirs is extremely important either economic or public health point of view because a great amount of visceras are rejected in order to maintain a low risk for human. However, due to work dynamics in slaughterhouses, it is usually difficult to uncover this etiology. In this study, we applied simple protocols to determine the final diagnosis and the etiology of such rejections. Over the course of a year, organs rejected during meat inspection were sampled from an ovine slaughterhouse in central Spain that slaughtered both sheep and lambs. The application of these protocols were very useful in the identification of bacterial agents involved in those rejections that clinically were compatible with enzootic pneumonia and caseous lymphadenitis as well as abscesses, among others. The regular application of these protocols would provide valuable information to establish control measures of those processes that reach to the slaughterhouses and in consequence to avoid they reach the human food chain.
Keywords
Slaughter, bacterial rejections, diagnose, ovine, Public health.
Citation
Vilallonga, D., Valcarcel, F., 2016. Improving the Diagnosis of Bacterial Rejections in Ovine Abattoirs by the Use of Simple Protocols. PSM Vet. Res., 01(1): 01-07.
Introduction
Bacterial processes affecting human and animals are usually multifactorial; however, there are one or two predominant agents involved in a great number of processes. For example, in the ovine enzootic pneumonia, excluding micoplasms, main bacterial agents are several strains of M. haemolytica, although from the lesions, occasionally, other organisms can be isolated: Pasteurella multocida, Bordetella parapertussis or Branhamella catharralis (Jones et al., 1979; Arrigo et al., 1984; Jubb et al., 1993; Hervás et al., 1996; Martin, 1996; Martin & Aitken, 2002). Similarly, Fusobacterium necrophorum is the most important agent involved in localised purulent processes in liver and lungs (Moreno, 2006) along with Dichelobacter spp. (Biberstein & Zee, 1994). The corinebacteria of intererst in meat inspection include Arcanobacterium pyogenes, agents of purulent infectious processes, and Corynebacterium pseudotuberculosis, which produces pseudotuberculosis or ovine caseous lymphadenitis (Moreno, 2006). The differential diagnostic with other pathologies that show up with abscesses of similar features is of high importance. Among them, infections caused by Staphylococcus aureus, Streptococcus spp. Arcanobacterium pyogenes (Burrel, 1980; Aleman & Spier, 2001; León et al., 2002) should be considered. The microbiological studies of the disease of abscesses, also known as enfermedad de Morel, have determined that the etiological agent that causes the condition is Staphylococcus aureus anaerobius (De la Fuente et al., 1985; De la Fuente, et al., 2011).
In this context, there is a clear interest in establishing the underlying bacterial etiology of the rejections. The aim of this study was to applicate several protocols and basic laboratory techniques to identify common bacterial agents involved and equipment.
Materials and Methods
From October 2010 to September 2011 in the central area of Spain. 40-50 lambs and 10-20 adult sheep older than two years of age were monthly sampled in a slaughterhouse in Madrid (Central Spain). A preliminary identification of each rejection were initially made by the official Veterinary inspector and immediately a portion of confiscated tissue was obtained with sterile procedures keeping samples at -20ºC until laboratory procedures.
According to the initial identification rejection samples: lesions compatible with enzootic pneumonia (105 cases in lambs and 17 in adults); with caseous lymphadenitis (24 cases in adults) and pulmonary abscesses; and with hepatic abscesses, caseous lymphadenitis, hepatitis and hepatic necrosis were processes following one of the protocols shown on Tables 1, 2 and 3, respectively. Any remaining bacterial species was rendered as unidentified and classified as “other”. Samples with no bacterial growth were classified as “no growth”.
In all cases bacterial identification entailed bacterial culture, bacterial staining from the lesion and the resulting culture, and biochemical testing (additional information may be seen in Vilallonga, 2013).
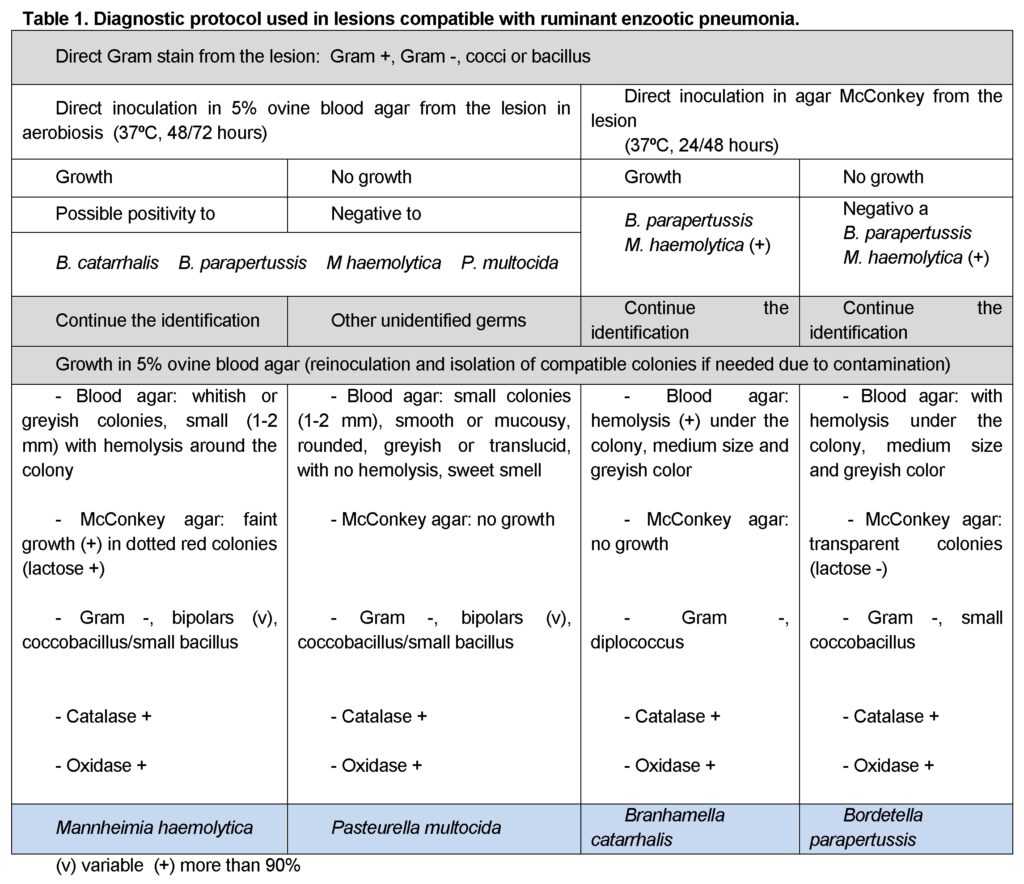
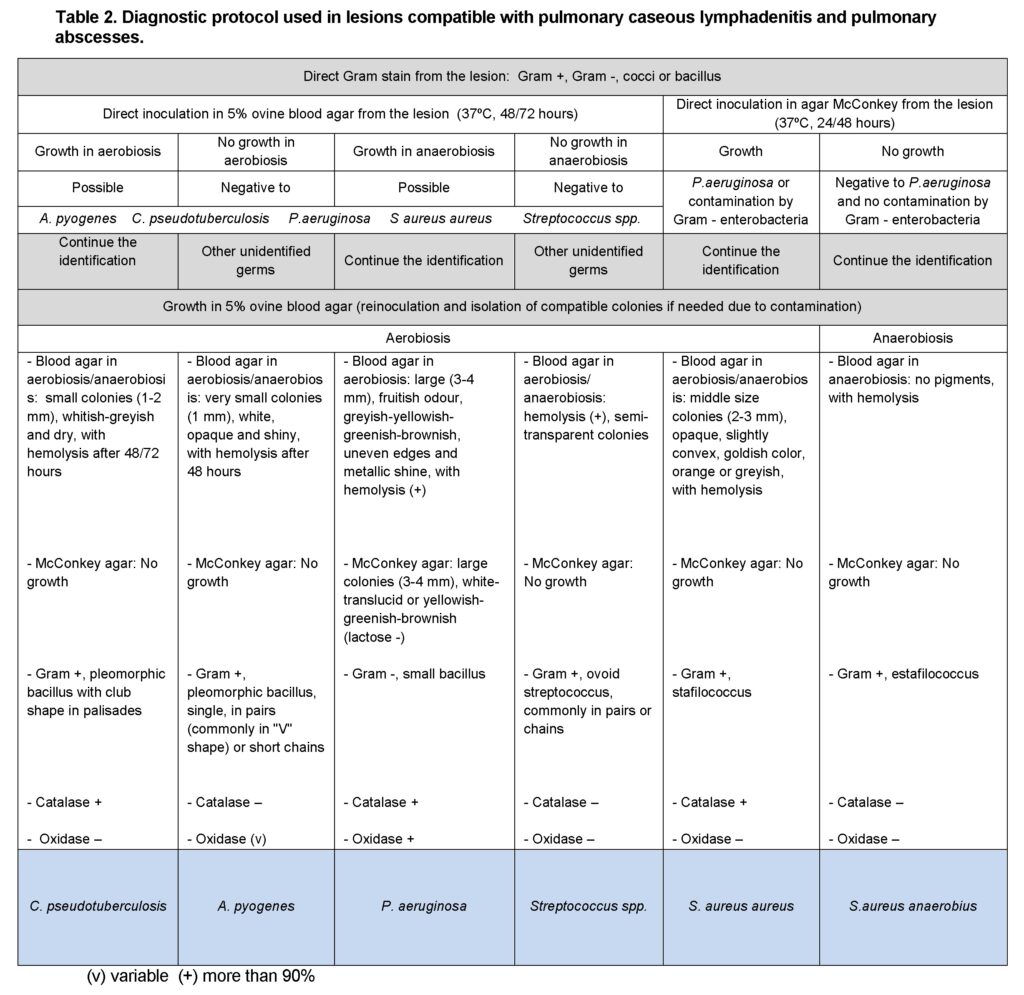
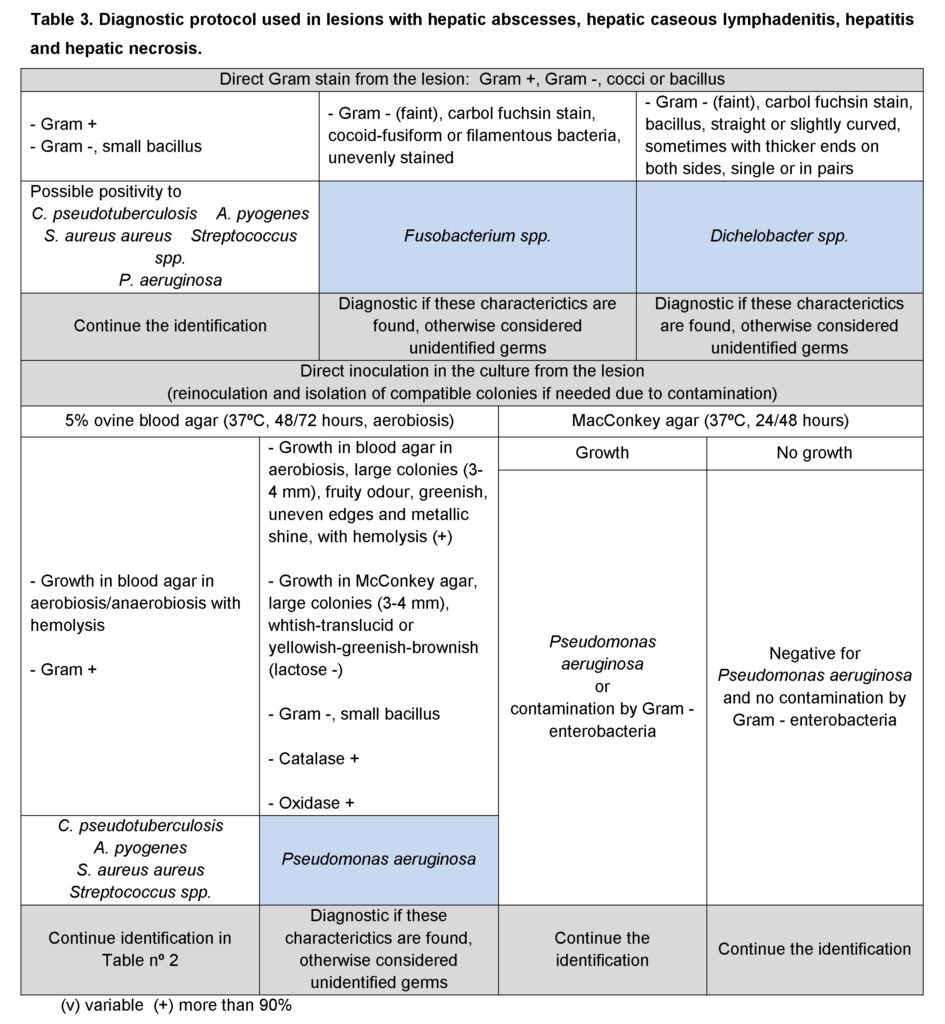
Results
A total of 2,429 animals were inspected and 577 organs were rejected, the 60.14% of which had a bacterial condition as the cause of the condemnation whilst the remainder of the rejections had either a parasitic origin or some other cause (38.13% and 1.73%, Valcárcel and Villalonga, 2015).
The number of cultures and isolations were variable due the irregular presence in the abattoir. So, we processed (lambs/adults) 105/17 cases of enzootic pneumonia; 0/24 of caseous lymphadenitis; 3/16 of other lung processes; 39/23 of liver abscess; 14/8 of other liver processes; 0/1 of abscesse disease; 13/0 of cisticercosis; and fnally 0/22 of hydatidosis. The bacterial identified in each rejection are shown in Table 4.
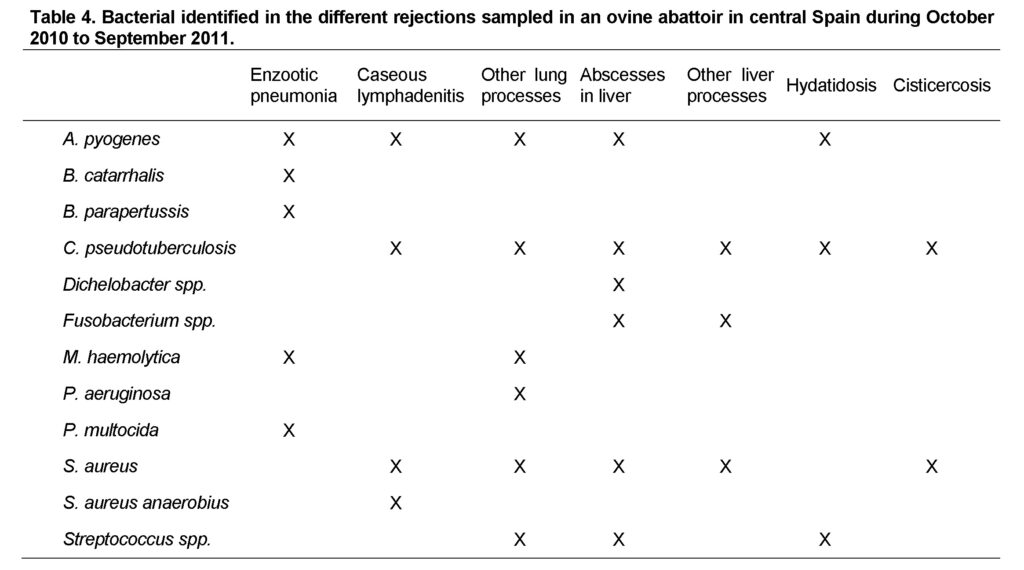
The main pathogen isolated from lamb pneumonic tissue was Manheimia haemolytica, which was present in 78.10% of the cultures, followed by Pasteurella multocida and Branhamella catarrhalis and, at much lower levels, Bordetella parapertussis and Arcanobacterium pyogenes.
Staphylococcus aureus was isolated in over half of lung abcess cultures, followed distantly by Fusobacterium spp., Streptococcus spp. and other unidentified pathogens. This pattern was similar in sheep and lambs, but in the case of adults, the unidentified microorganisms had a higher percentage of prevalence (17.95% and 34.78%, respectively).
Among the microorganisms isolated from the lesions caused by caseous lymphadenitis, Corynebacterium pseudotuberculosis was prominent and found in 100% of lesions consistent with caseous lymphadenitis, with occasional contamination by Staphylococcus aureus or other germs. We found one specific disease associated with caseous lymphadenitis, the abscesses disease, in one case from the spring. The culture of this condemnation rendered growth for Staphylococcus aureus anaerobius and C. pseudotuberculosis.
S. aureus was isolated in over half of hydatid cysts, followed by other pyogenic bacteria such as Streptococcus spp., A. pyogenes and Corynebacterium pseudotuberculosis. Similarly, a quarter of lambs with abscesses also had cysticercosis, and 70% of the lesions caused by cysticercosis showed bacterial growth Staphylococcus aureus.
Discussion
Despite the variety of reasons for condemnation, only a few bacterial diseases or processes – enzootic pneumonia, caseous lymphadenitis and abscesses in this study— are found in most rejections, as has been previously described (Jepson and Hinton; 1986; Vilallonga, 2013).
Enzootic pneumonia.The identification of bacteria by lamb pneumonic tissue culture appears to be variable. Our findings of M. haemolytica followed by P. multocida and B. catarrhalis and, at much lower levels, B. parapertussis and A. pyogenes are similar to those found by other authors (Pinto, 2011; Arrigo et al., 1984). Interestingly, although there is generally less isolation in adults than in lambs, isolates of M. haemolytica remain the most frequent in lesions caused by enzootic pneumonia, followed by P. multocida and B. catarrhalis.
Abscesses.The high presence of S. aureus, Streptococcus spp., A. pyogenes and C. pseudotuberculosis in cultured hydatid cysts and S.aureus in cysticercosis samples suggests a possible association between the presence of metacestodosis and liver and lung abscesses. The other isolates obtained from abscess cultures confirm the polymicrobial nature of suppurative infections, as demonstrated by the disparity found in the literature. For example, our data agree with those of Scanlan (1991) and Quinn et al. (2002), which highlight the isolation of two or more species of facultative anaerobes and/or obligate anaerobic, often including F. necrophorum and Bacteroides spp.
Fusobacterium spp. and Dichelobacter spp. constitute more than half of anaerobes isolated from mixed opportunistic infections (Moreno, 2006). However, the difficulties in their isolation and identification can lead to an underestimation of their involvement (Quinn et al., 2002). This may have occured in the present study because the method employed for detection of these bacteria was not sufficiently specific for their complete identification.
Caseous lymphadenitis. C. pseudotuberculosis was isolated in all caseous lymphadenitis lesions, with S. aureus or other pathogens occasionally growing concomitantly. These results are in agreement with other studies that reported that both species were the most frequent isolates (Brown et al., 1987; Ben Saïd et al., 2002; Chikhaoui and Khoudja, 2013). Only abscess disease was concomitant to caseous lymphadenitis, that is; because it only manifested once, it does not appear to be relevant but rather a coincidential circumstance. Abscess disease has proven to be an uncommon finding and complicates the diagnosis during post-mortem inspection because it presents no characteristic lesion beyond a purulent abscess.
Other liver and lung processes. This group of lesions was used as a way to arrange a diversity of low incidence pathologies found during the research like necrosis, adhesions, hepatitis or pneumonitis. There was no clear pattern on their presentation probably due to their difference in origin. The bacterial isolations showed that most of these lesions were pyogenic in nature.
Conclusion
The bacteriological protocols proposed seemed to be be a practical, cost-effective and useful tool in the primary identification of common bacterial species involved in the principal pathologies found in slaughtherhouses. The results clearly show that the vast majority of the ovine bacterial pathologies are caused by a handful of bacterial species: M. haemolytica, P. multocida, A. pyogenes, C. pseudotuberculosis and S.aureus. Hence, the bacterial protocols mentioned above could be used extensively as a way to assess and better understand the situation of the national ovine livestock regarding the most common diseases found in this species. This not only would allow the implemmentation of specific animal health policies in order to decrease the prevalence of these pathologies and further investigate their epidemiology but also increase the quality of meat inspection procedures. Another consideration to bring is the fact that the protocols proposed imply the use of very simple and affordable laboratory equipment which would help the application of this system in undeveloped countries and satisfy the aforementioned goals.
Acknowledgement
This study was supported by the Spanish Research Project RTA2010-00094-C03-03.
Conflict of Interest
The authors confirm that this article content has no conflict of interest.
References
Aleman, M., Spier, S.J., 2001. Corynebacterium pseudotuberculosis infection. Large Animal Internal Medicine. Smith P.B. Mosby, St. Louis, 1078-1084.
Arrigo, J.L., Terzolo, H. R., Casaro, A., Villar, J., 1984. Neumonía enzootica ovina. Rev. Med. Vet., 2: 74- 80.
Ben Saïd, M.S., Ben Maitigue, H., Benzarti, M., Messadi, L., Rejeb, A., Amara A., 2002. Epidemiological and clinical studies of ovine caseous lymphadenitis. Archives de l’Institut Pasteur de Tunis, 79 (1-4): 51-7.
Biberstein, E.L., Cheng Zee, Y., 1994. Tratado de Microbiología Veterinaria. Acribia, Zaragoza.
Brown, C.C., Olander, H.J., Alves, S.F., 1987. Synergistic hemolysis-inhibition titers associated with caseous lymphadenitis in a slaughterhouse survey of goats and sheep in Northeastern Brazil. Canadian J. of Vet. Res., 51 (1): 46-9.
Burrel, D.H., 1980. Caseous lymphadenitis in goats. Aust. Vet. J., 57, 105-110.
Chikhaoui, M., Khoudja, F.B. 2013. Clinicopathological investigation on caseous lymphadenitis in local breed sheep in Algeria. Trop. Anim. Health Prod., 45 (7): 1641-3. doi: 10.1007/s11250-013-0410-7.
De la Fuente, R., Ballesteros, C., Bautista, V., Medina, A., Orden, J.A., Domínguez-Bernal, G., Vindel, A. 2011. Staphylococcus aureus subsp. anaerobius isolates from different countries are clonal in nature. Vet. Microbiol., 150: 198-202.
De la Fuente, R., Suárez, G., Schleifer, K.H., 1985. Staphylococcus aureus subp. anaerobius subsp. nov., the causal agent of abscess disease of sheep. Int. J. Systematic Bacteriol., 35: 99-102.
Hervás J., Méndez A., Gómez-Villamandos J.C., Villalba E., Díaz E., Cano T., Carrasco L., Padró J.M., Fernández A., Sierra M.A. (1996). Etiologic and pathologic study of respiratory disease in lambs from intensive breeding facilities in southern Spain. Zentralbl Veterinarmed B, 43: 221-31.
Jepson, P.G., Hinton, M.H., 1986. An inquiry into the causes of liver damage in lambs. Vet. Rec., 118: 584-587.
Jones, G.E., Buxton, D., Harker, D.B., 1979. Respiratory infections in housed sheep, with particular reference to mycoplasma. Vet. Microbiol., 4: 47-59.
Jubb, K.V.F., Kennedy, P.C., Palmer, N., 1993. Pathology of Domestic Animals. Fourth Edition. Academic Press, Orlando, Florida.
León-Viscaino, L., Garrido, F., González M., Cubero, M.J., 2002. Anatomía patológica de la pseudotuberculosis. Rev. Ovis. http/ www.exopol.com/ circulares/205.htmi.
Martin, W.B., 1996. Respiratory infections of sheep. Comparative Immunology, Microbiology, Infectious Diseases, 19: 171-179.
Martin, I.D., Aitken, W.B., 2002. Enfermedades de la oveja. 2nd ed. Acribia, Zaragoza.
Moreno, B., 2006. Higiene e Inspección de carnes. Díaz de Santos, Madrid.
Pinto, C.E., 2011. Epidemiología molecular de las poblaciones bacterianas de Mannheimia haemolytica y Pasteurella multocida asociadas a la presencia de lesiones neumónicas en corderos en matadero. Tesis Docotoral. Universidad Complutense de Madrid.
Quinn, P.J., Carter, M.E., Markey, B., Carter, G.R., 2000. Clinical veterinary microbiology. Mosby, Edimburgo.
Scanlan, CH.M., 1991. Introducción a la Bacteriología Veterinaria. Acribia, Zaragoza.
Vilallonga, D., 2013. Estudio de la etiología e impacto económico de los decomisos en un matadero de ovinos. Doctoral Thesis. University of Extremadura, Cáceres, Spain.





