Morphological and Biochemical Characterization of Xanthomonas axenopodis pv. citri Isolates Causing Citrus Canker Disease in Pakistan
1Department of Plant Pathology, University of Agriculture, Faisalabad, Pakistan.
2Plant Protection Division, Nuclear Institute for Agriculture and Biology (NIAB), P.O. Box 128, Jhang Road, Faisalabad, Pakistan.
3Plant Breeding and Genetics Division, Nuclear Institute for Agriculture and Biology (NIAB), P.O. Box 128, Jhang Road, Faisalabad, Pakistan.
*Corresponding author:
Kamran Saleem;Email:
kamranniab@gmail.comViews 3368
Abstract
Citrus canker inflicted by bacterial pathogen, Xanthomonas axonopodis pv. citri (Xac) is very destructive disease. It is distributed worldwide and declared as quarantine pathogen due to its ability of damage when established. In the current perusal, 25 Xac isolates representing Faisalabad and Sargodha were characterized for their biochemical and morphological features. The pathogen was isolated and identified on the basis of microscopy, morphological characters and biochemical characters. After isolation and purification of pathogen, 25 isolates were used for biochemical characterization of pathogen. Six biochemical tests were used for confirmation of pathogen. In Gram’s staining, Kovac’s oxidase and Arginin dihydrolase tests, all Xac isolates showed negative results while positive results were observed in case of starch hydrolysis, tween 80 hydrolysis and gelatin liquification tests. Pathogenicity test was also performed and on all citrus varieties, development of typical symptoms was observed which confirmed the pathogen. Although, isolates were diverse and vary in level of aggressiveness. Albeit, this study is baseline for formulating appropriate management strategy in controlling citrus canker disease.
Keywords
Biological control, antagonistic microorganisms, plant pathogenic fungi, inhibition zone.
Citation
Jabeen, T., Arshad, H.M.I., Saleem, K., Ali, S., Ullah, E., Naureen, S., Babar, M.M., 2016. Morphological and Biochemical Characterization of Xanthomonas axenopodis pv. citri Isolates Causing Citrus Canker Disease in Pakistan. PSM Microbiol., 01(1): 10-17.
Introduction
Citrus (Citrus sinensis) is a very important fruit and export commodity of Pakistan with total production of 2334 thousand tons (GOP, 2012-13) while farming area is 195,000 ha (FAO, 2012). Biotic and abiotic stresses hampered its production throughout the world and citrus canker among all is the most devastating disease (Burhan et al., 2007). Although the tree is affected by various diseases like citrus greening, quick decline, citrus slow, citrus wither tip and citrus gummosis, but citrus canker caused by Xac (Xanthomonas axenopodis pv. citri) is one of the most threatening of citrus diseases (Das,2003). Millions of trees were burnt in Florida as eradication of Xac and afterwards declared as important quarantine pathogen in many countries. Xac caused damage in citrus fruits both in term of yield and quality but extents of damage fluctuate with existing environments, species and varieties (Das and Singh, 2003). Citrus canker disease was first reported from Punjab and then after establishment become major disease in all citrus growing areas of Pakistan and permanent problem of citrus grower in this area.
Xac is also notorious in terms of its wide host range wherein it infects all citrus species and cultivars. Grapefruits and sweet orange were highly susceptible while sour orange exhibits some extent of resistance (Civerolo, 1984). The symptoms of citrus bacterial canker include abrasions spreading on all foliar parts of tree. Canker is often characterized by rounded necrotic spots on leaves and rind-blemishing fruit. Lesion typically starts with pinpoint which may attain size of 2-10mm and within few days lesion turn into pustule first on underside of the leaf and later on upper side. These pustules become corky with raised margins and surrounded by yellow halo spot. Presence of such yellow water soaked margins around the necrotic corky spot is characteristic symptoms of citrus canker. However, type and severity of symptoms depend upon the type of cultivar, environmental conditions, age of the plant and type of citrus canker pathogen prevalent in that geographical area. Millions of bacteria are produced per lesion depending upon the level of resistance in citrus cultivar and lesion size (Graham et al., 1992). Five forms of the citrus canker have been documented as, cankers “A”, “B”, “C”, “D”, and “E” based upon host range, level of aggressiveness and geographical origin (EPPO/CABI., 1997; Schaad et al., 2005). The most common form, first reported in Florida, is Canker A or A-strain (Asiatic canker), which is most threatening of the citrus canker strains (Schubert et al., 2001). Most scientists have documented this strain as citrus canker disease.
All five forms of Xac can be differentiated through pathogenicity and growth on differential media. In addition, biochemical features may be differetnt among isolates of Xac. Citrus canker bacterium is a gram negative rod having single polar flagellum. Additionally, Xac can produce proteolytic enzymes hydrolyzing casein, gelatin, esculin and starch and produces ammonia and hydrogen sulfide from peptone sources (Whiteside et al., 1993; Goto, 1992). These morphological and biochemical features of Xac can be used for identification of Xac isolates collected from different locations. So objective of this study is to characterize the Xac isolates on the basis of morphology, pathogenicity and biochemical features.
Materials and Methods
Collection of samples
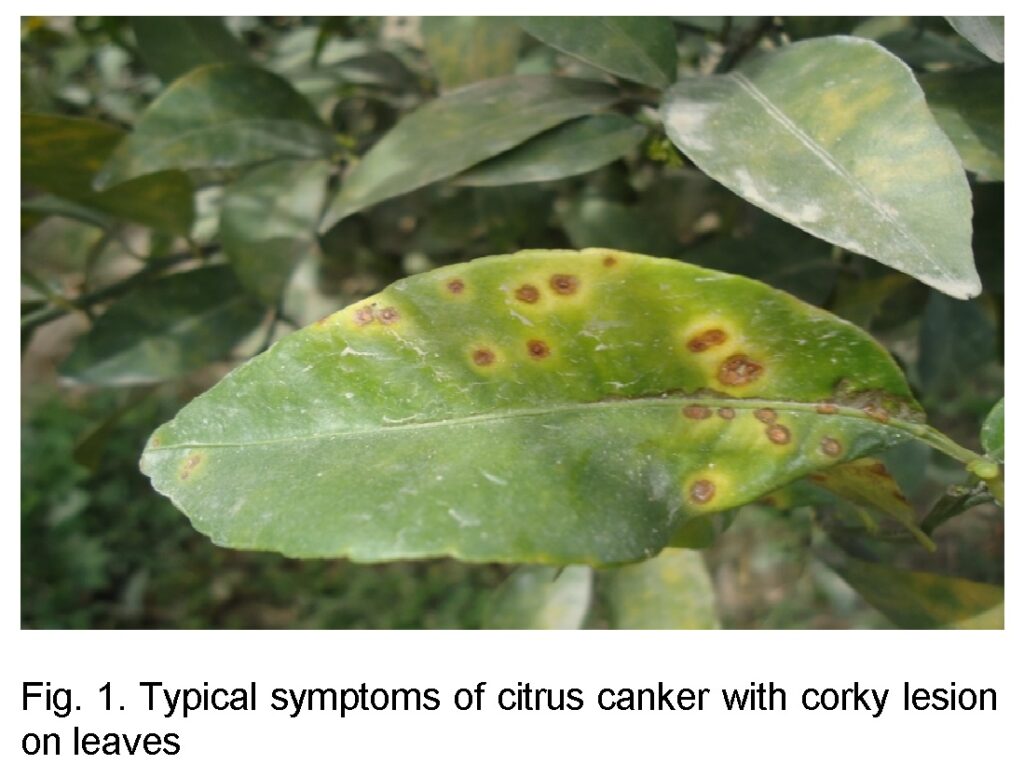
Leaf samples of citrus plant were collected on the basis of typical symptoms (Figure 1) from Citrus orchard, NIAB Faisalabad, Citrus orchard, PARAS institute of University of Agriculture, Faisalabad and Citrus orchards, Sargodha. 10 infected leaves were collected in Plastic envelops and brought in Fungal and Bacterial diseases laboratory of Plant Protection Division, NIAB Faisalabad for further processing.
Isolation and Purification of Pathogen
Nutrient Agar media (Agar. 15g, Nutrient Broth. 13g, Distilled water. 1000ml) was prepared by mixing the Nutrient Broth and Agar in water. Leaf tissues showing typical citrus bacterial canker were cut down into 2 X 2 mm discs. The samples were surface sterilized with 70% ethanol (10 ml of 70 % ethanol prepared using 7ml of ethanol and 3 ml of water using micropipette) in eppendorf tubes and by giving subsequent washing with distilled water in eppendorf tubes. The diseased samples were placed in distilled water for 15 minutes. Direct streaking were carried out by taking a sterilized wire platinum loop full of bacterial suspension and streaked out on nutrient agar (NA) medium and similarly the direct sampling was done by placing infected tissues discs on the nutrient agar medium under aseptic conditions and petri plates were incubated at 27°C for 24 hours.
The single purified colonies of that bacterium was streaked with the help of a sterilized wire loop to nutrient agar slants (in duplicate) and were incubated at 27°C. One set of slants having purified cultures were preserved at – 20°C and other were kept in refrigerator for further use.
Biochemical characterization of Xanthomonas axonopodis pv.citri
Plant pathogenic bacteria contain diverse cellular and biochemical metabolism which may be specific for each pathogen. Many biochemical tests were developed as identification marker and also facilitate in determining biochemical characters of bacteria. Biochemical tests following Bergey’s Manual of Determinative Bacteriology (Bergey, 1984; Iqbal et al., 2015; Yunus et al., 2016) were performed in current study.
Gram’s staining
Gram’s reagents were made using crystal violet, Lugol’s iodine, Acetone (pure) and counterstain Safranin. Xac isolates were made by taking a pure culture with the help of sterilized inoculating loop and make smear on clear slide with distilled autoclaved water. A drop of crystal violet was placed on smear and kept for 30 seconds followed by washing with sterilized water. Afterwards, drop of Lugol’s iodine was placed (30s) followed by washing with the DAW and then permanently washed with pure acetone. Finally a drop of Safranin was added, kept for 30s and washed with the water. The mounts were dried using blotter paper, a drop of Canada balsam was placed on the stained and observed under microscope at 100 X.
Starch Hydrolysis Test
Nutrient agar medium was prepared by dissolving 15g of agar and 13g of nutrient broth in distilled water (1L). The rice starch of 2g was dissolved in 10 mL of water in a separate flask. The starch was added to the molten NA with continues stirring on the hot plate. The media was autoclaved at 121°C and 15psi for 15 minutes. The basal medium was dispensed into sterilized petri plates. Each isolate was transferred into the medium under laminar flow cabinet and the plates were incubated at 27°C for seven days. The plates were allocated with 3% Lugol’s iodine after scraping the Xac culture/without scraping on the media (Cowan, 1974).
Tween- 80 Hydrolysis Test
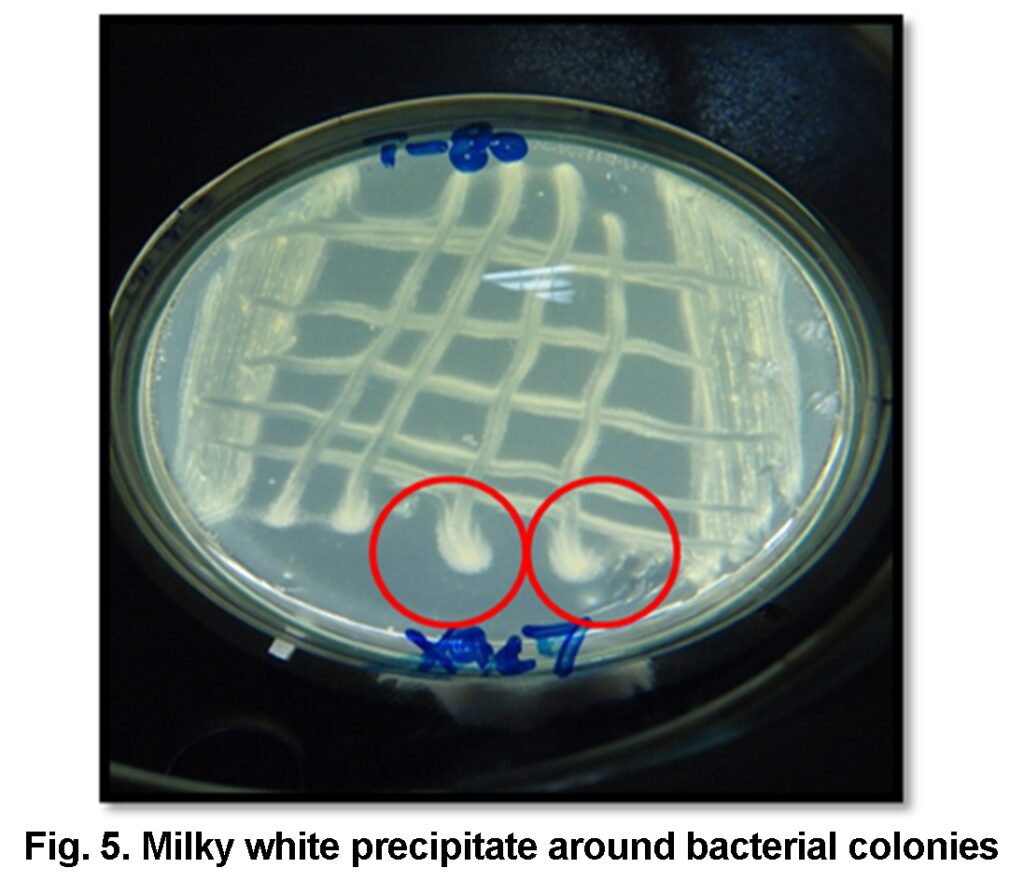
pH 7.4), autoclaved and 1% of Tween 80 was added to the molten media. After pouring media into the sterilized petri plates isolates of Xac was streaked and incubated at 27°C for seven days. Presence or absence of Opaque milky precipitate / milky crystal formation around bacterial colonies is indication of positive or negative test respectively (Sierra, 1957).
Kovac’s Oxidase Test
For this test 1% Kovac’s reagent was poured on the center of Whatman filter paper No.1 and all Xac isolates were multiplied in Nutrient Agar. An inoculum of bacteria was gently rubbed on the filter paper with Platinum loop. The isolates were oxidase positive if a purple color developed within 10-60 seconds but negative in the absence of coloration or if color developed after 1 minute (Kovac, 1956).
Gelatin Liquefaction Test
Gelatin medium (Beef extract 3g , Peptone 5g, Gelatin 120g in 1000ml water) was prepared, poured into 5mL test tubes, plugged and autoclaved. The 24hours old Xac culture of each isolate was inoculated and incubated at 27°C. After 72 hours, tubes were placed at 4°C for 30 minutes prior to recording of results. The same procedure was pursued after 7, 14 and 21 days. If the gelatin was flowed easily when test tube was gently tipped it’s indicate hydrolysis of gelatin had taken place by bacteria, while unhydrolysed gelatin was unable to flow (Cowan, 1974).
Arginine Dihydrolase Test
Thornley’s medium was prepared by adding all the ingredients into DAW and boiled to dissolve the agar. pH was adjusted at 7.2, then medium was poured into the screw-cap tubes and autoclaved at 12°C and 15psi for 10 minutes. Tubes were stab inoculated from the 24 h old colonies, covered with 1cm paraffin oil (sterilized) and incubated at 27°C for three days. The change in color to red showed positive results.
Pathogenicity Test
Koch’s postulates are the baseline for declaring any microorganism as pathogen and for Xac this pathogenicity test need to be performed. For pathogenicity test, sweet orange was used while for aggressiveness, panel of indicator hosts such as Duncan grapefruit, red blood and kinnowwere inoculated with purified isolates of Xac. For pathogenicity test, all isolates of Xac were grown in liquid media or bacterial cells were scraped off from a freshly streaked agar plate and suspended in sterile distilled water for inoculation into citrus. The concentration was adjusted to 108 CFU mL−1 and leaves were inoculated through pin prick method and labeled with the respective isolate. Pathogenicity and aggressiveness of all isolates were determined based on already described disease rating scale (Ijaz et al., 1999).
Number of lesions (%) = Total lesions on leaves / Total leaves x100
Results
Isolation and Purification of Xanthomonas axonopodis pv.citri
The unique opaque yellow colonies of Xac exude out from leaves placed on nutrient agar plates (Figure 2) after 48 hours of incubation at 300C temperature. Yellow raised colonies are due to Xanthin produced by members of genus Xanthomonas. Along with visual observation, the colony color of all Xac isolates varied from yellow to light yellow. The size and shape of colonies was small to medium, convex and mucoid (Table 1).
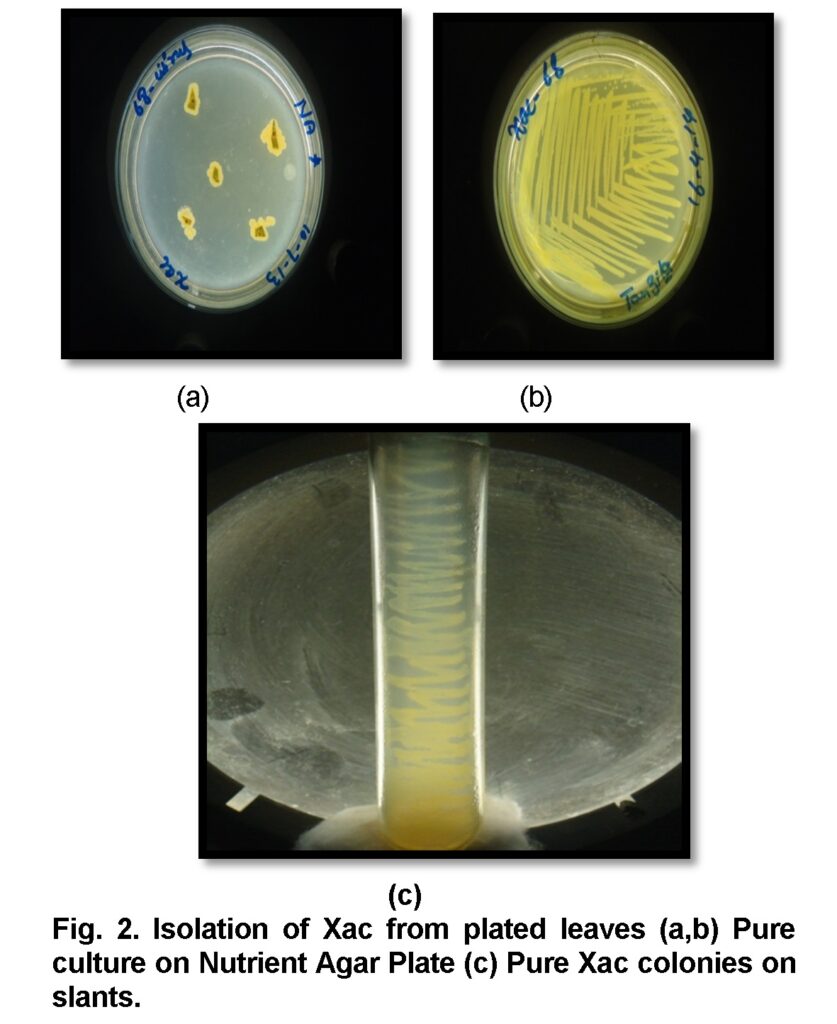
Biochemical characterization of Xac
All the isolates of the Xac responded negatively when observed under light microscope at 100x using oil immersion. Some isolates appear as red and other as pink and were found to be gram negative because cell wall did not retain color and washed away when treated with ethanol. Cell morphology was rod shaped and pinkish in color when stained with counter strain safranin (Figure 3).
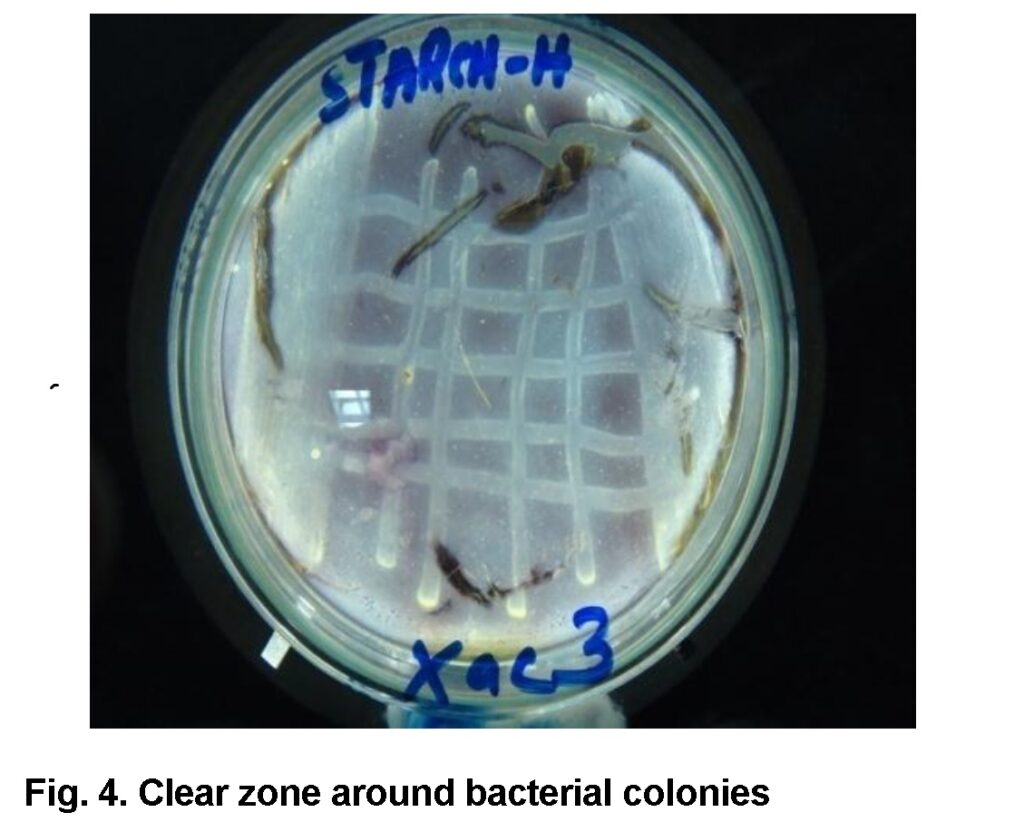
Starch hydrolysis test
In this test, clear golden zone was observed around bacterial colonies on starch agar when stained with Lugol’s iodine after 7 days of incubation at 27°C (Figure 4). The bacteria hydrolyzed the starch and exhibit unique zone. All of the Xac isolates showed positive test.
Tween 80 hydrolysis test
All the bacterial isolates hydrolyzed the tween 80 that is indicated by the presence of milky white precipitate/opaque zone around the bacterial colonies after 3rd day of incubation at 27°C. It’s the unique feature of many bacteria under genus Xanthomons and all isolates were found positive for this test (Figure 5).
Kovac’s oxidase test
The principal of Oxidase test is based on presence or absence of cytochrome oxidase, which is the unique feature of saprophytic bacteria. Appearance of purple/dark blue color within 10-60 seconds is an indication of positive test. All the Xac isolates were oxidase negative (Figure 6).

Gelatin Liquefaction test
The principal of this test is the conversion of semisolid gel into liquid due to hydrolysis by bacteria. If gelatin medium starts to flow upon the tilting of test tube showed the positive test. All the Xac isolates of were able to liquefy the gelatin medium (Fig.7) as compared to control.

Arginine dihydrolase test
In principal of this test, Arginine can be degraded into ornithine through an enzymatic reaction and as a result produces ATP and NH3. This test is also used for identification of certain pathogens which are able to grow under anaerobic conditions due to this mechanism. As a result of the ornithine production, ADP and carbamate kinase, ATP, CO2 and NH3 are formed, and the medium turns alkaline. Phenol red color appears if bacteria utilize arginine depending on the pH level. The Xac isolates inoculated by stab method were unable to utilize arginine (Figure 8) and considered as negative for Arginine dihydrolase test.

Pathogenicity test
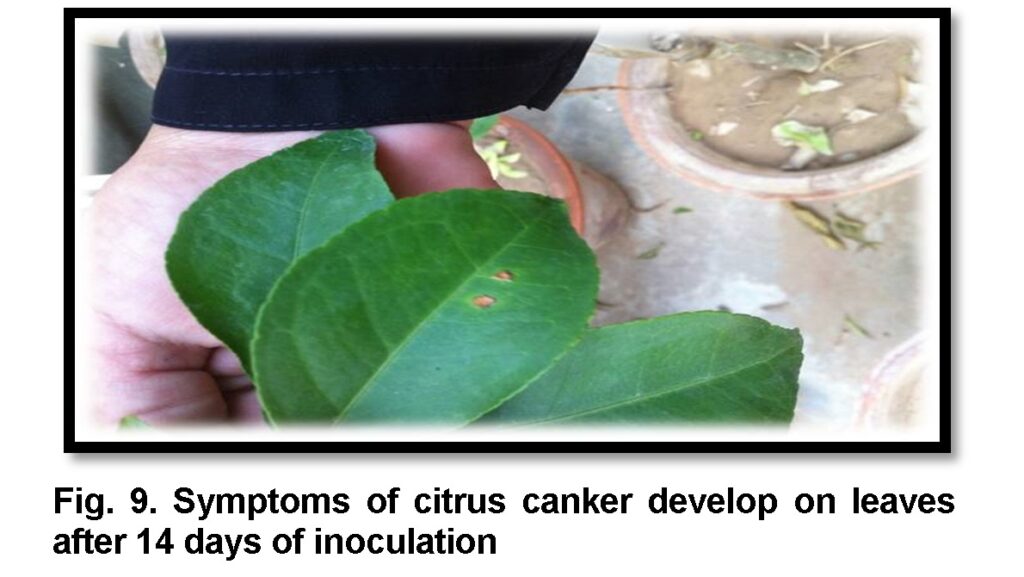
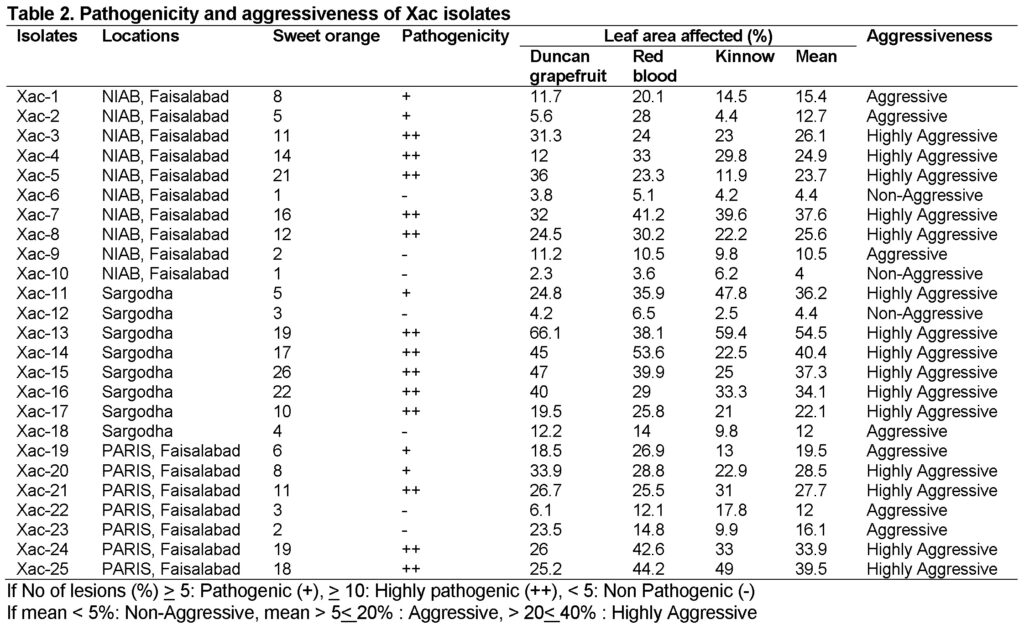
Pathogenicity test was performed using pin prick method on sweet orange while for aggressiveness grapefruit, red blood and kinnow leaves were inoculated. After 14 days of inoculation symptoms initiated as spots and eventually converted into pustules with a raised margin surrounding a halo spot. Brown color callus were also observed on the lower side of leaves around inoculation site after 14 days of incubation at 28°C (Fig 9). All the isolates were found pathogenic on sweet orange except seven. These seven were observed in all three locations. Xac-15 from Sarhoda was highly pathogenic and produced maximum lesions on sweet orange followed by Xac-16 and Xac-5. While testing aggressiveness on three hosts, all citrus varieties behave differently against Xac isolates. Xac-13 from Sargodha was found highly aggressive and confer maximum disease on all three hosts overall and among 16 highly aggressive Xac isolates. Only three isolates: Xac-6, Xac-10 and Xac-12 were non aggressive on panel of hosts because produce very minor spots of chlorosis which may be due to host defense response (Table 2).
Discussion
Purpose of this study was to isolate and confirm the bacterium Xac, isolates of Xanthomonas axonopodis pv.citri collected from different areas of NIAB, Faisalabad and from citrus orchards of District Sarghoda.
The genus Xanthomonas bacteria are straight, Gram-negative rods, mostly yellow-pigmented with a polar flagellum. They are strictly aerobic chemo organotrophs, and most of the strains are phytopathogenic.
The leave samples on Nutrient agar plates showed the bacterial colonies after 48 hours of incubation at 27 °C temperature. The visual observation was carried out to identify the colony morphology. The colony colour was yellow. The size and shape of colonies was small to medium, convex and mucoid. The staining reaction was observed under microscope and bacteria were found to be gram negative. These results were also supported by (Breed et al., 1989). Bacterial colonies were identified through it were of small, medium and large sized and pink in colour under microscopic view. The results are in line with previous study (Arshiyaet al., 2014) in which morphological, biochemical and pathogenicity tests were performed to identify and characterize the strains of bacteria causing citrus canker. All the biochemical tests confirmed the pathogen.
Pathogenecity test was carried out for confirmation of pathogen and found different pathogenic behavour on panel of host. Previously pathogenicity of purified Xacstrains was evaluated on detached grapefruit leaves cv. Duncan (Al-Salehet al., 2014). Albeit, this study is baseline for formulating appropriate management strategy in controlling citrus canker disease which has already become a threat to the citrus production in Pakistan.
Conclusion
Combativeness of Xac isolates in this study helped to use suitable management practices at different locations. Morphological and biochemical characterization is the baseline to perceive pathogen population and biological phenomenon, which enhances aggressiveness of pathogen. Molecular studies should be carried out to better understand the mechanism of pathogenicity.
Acknowledgement
Authors are very grateful to Mustansar Mubeen for collecting and providing the disease samples of citrus from Sargodha district. We also acknowledge the assistance of Umra Sadaf during isolation and purification of pathogen.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Ahmed, R., Khan, H.U., 1999. Citrus decline: Problems and Progress in the Punjab. A Review: Proc. 2nd Nat. Conf. Pl. Path. Univ. Agri., Faisalabad. pp. 20-22.
Al-Saleh, M.A., Widyawan, A., Saleh, A., Ibrahim, Y.E., 2014. Distribution and pathotype identification of Xanthomonas citri subsp. citri recovered from south-western region of Saudi Arabia. Afr. J. Microbiol. Res., 8(7): 673-679.
Arshiya, M., Suryawanshi, A., More, D.,Baig, M.M.V., 2014. Repetitive PCR based detection of genetic diversity in Xanthomonas axonopodis pv. citri Strains. J. Appl. Biology. Biotech., 2(1): 017-022.
Bergey, S.A., 1984. Bergey, Manual of Determinative Bacteriology, 9th edition, Williams & Wilkins., Philadelphia.
Breed, R.S., MurryE.G.D.,Smith, N.R., 1989. Bergey’sManual of Systemic Becteriology. Eds. S.T.Williums.
Burhan, M., Sahi, S.T.,Ahmad, S., 2007. Screening of citrus cultivars for source of resistance against citrus canker under field conditions. Pak. J. Bot. 39: 1867-1871.
Civerolo, E.L., 1984.Bacterial canker disease of citrus.Anoverview.Proc. Int. Soc. Citric.390-394.
Cowan, S.T., 1974. Manual for the identification of medical bacteria.CambridgeUniversityPress, Great Britain. 238.
Das, A.K., 2003. Citrus canker – A review. J. Appl. Hort. 5(1):52-60.
Das, A.K.,Singh, S., 2003. Integration of chemicals and cultural practices for management of bacterial canker (Xanthomonasaxonopodispv. citri) in acid lime (Citrus aurantifolia). Ind. J. Agric. Sci. 73: 570-571.
EPPO/CABI, 1997.Xanthomonasaxonopodispv.citriIn: Quarantine Pests for Europe. 2nd ed. Smith,McNamara, Scott & Holderness. CABI International, Wallingford, UK.
FAO, 2012. Food and Agriculture Organization. www.fao.org.
GOP, 2013.Economic survey of Pakistan.Division of economic affairs.
Goto, M., 1992. Citrus canker. In: Plant Diseases of International Importance. Vol. III. J. Kumar, H.S. Chaube, U.S. Singh and A.N.Mukhopadhyay. (Eds.) Prentice-Hall, Englewood Cliff, NJ.170-208.
Graham, J.H., Gottwald, T.R., Riley, T.D.,Achor, D.,1992. Penetration through leaf stomata and strains of Xanthomonascampestris in citrus cultivars varying in susceptibility to bacterial diseases. Phytopathology, 82, 1319–1325.
Ijaz, M., Ishfaq, M., Hafiz, M.I., Chaudhary, GA., 1999.Screening of citrus varieties against citrus canker (Xanthomonascampestrispv. citri) under rainfed climate of Pothwar. International Journal of Agriculture and Biology, 1(3): 108-109.
Iqbal, M.N., Anjum, A.A., Ali, M.A., Hussain, F., ALI, S., Muhammad, A., Irfan, M., Ahmad, A., Irfan, M. and Shabbir, A., 2015. Assessment of microbial load of un-pasteurized fruit juices and in vitro antibacterial potential of honey against bacterial isolates. Open Microbiol. J., 9: 26-32. DOI: 10.2174/1874285820150601E001.
Kovac, N., 1956. Identification of Pseudomonas pyocyaneaby oxidase reaction.Nature, 4: 535-703.
Sawarmura, M., 2000.Aroma and functional properties of Japanese Yuzu (citrus junolanaka) essential oil. Aroma Res. 1(1):14-19.
Schaad, N.W., Postnikova, E., Lacy, G., Sechler, A.J., Agarkova, I., Stromberg, P., Stromberg, V.Vidaver, A., 2005.Reclassification of xanthomonas species pathogenic on citrus. Systematic and Applied Microbiology, 28: 494-518.
Schubert, T.S., Rizvi, S.A., Sun, X., Gottwald, T.R., Graham, J.H.,Dixon, W.N., 2001. Meeting challenge of eradicating citrus canker in Florida-again. Plant Dis., 85:340-356.
Sierra, G., 1957. A simple method for the detection of lipolytic activity of microorganism and some observations on the influence of contact between cells and fatty substrates. Antonie Van Leeuwnhook. 23: 15-22.
Whiteside, J.O.,Garney,S.M., Timmer, L.W., 1993. Compendium of citrus diseases. The American Phytopsathological Society.p: 80.
Yunus, F.N., Kanwal, F., Rashid, F., Ashraf, A., Iqbal, M.N., Xiao, S., 2016. A Comparative Study on Isolation and Identification of Bacillus thuringiensis from Different Localities of Gujranwala City. PSM Biol. Res.,01(1): 34-38.