Molecular Characterization of Hydatid Cyst from Egyptian one Humped Camels (Camelus dromedaries)
1Department of parasitology, Faculty of Veterinary Medicine, Souhag University, Souhag, Egypt.
2Department of Parasitology, Animal Health Research Institute (AHRI), Shebin El koom, Menoufiya, Egypt.
*Corresponding author:
Amer R. Abdel Aziz;Email:
amerrageb77@yahoo.comViews 2749
Abstract
Hydatidosis or cystic echinococcosis (CE) is caused by Echinococcus granulosus (EG) complex, which infects humans especially children and several species of wild and domestic animals including camels, cattle, equines, sheep and goats. Infection come from the consumption of Echinococcus granulosus eggs with contaminated food, and therefore the syndrome is of public health significance. There are several genotypes nearly ten from genotype 1 to genotype 10, which are found all over the world based on genetic diversity. Several studies are conducted on the genetic diversity of hydatid cyst on Egyptian camels (Camelus dromedaries) at Qalyubia Governorate, Egypt, and this study is the continuation of previous works. In this study a total of 55 hydatid cysts samples were collected from the lungs of camels admitted to abattoir of Qalyubia Governorate, Egypt. Brood capsules, free solitary scolices, and sediment of hydatid fluid, were the source of DNA, which was extracted by the commercial kit. For polymerase chain reaction (PCR) amplification, two gene primers, the cytochrome C oxidase subunit 1 (cox1 gene), and the mitochondrial NADH dehydrogenase subunit 1 (NADH1 gene) were used and the partial sequences obtained were analyzed by gene sequencing and then phylogenetic analysis was carried out. Results of this study showed that 90.5% (n = 49/55) of the samples were identified as Echinococcus canadensis genotype 6 (G6), and 9.5 % (n=6/55) were identified as Echinococcus ortleppi (G5) which newly imported from Sudan for slaughtering. The presence of Echinococcus ortleppi of the cattle isolate (G5) in the Egyptian camels was concluded for the first time.
Keywords
Echinococcus ortleppi, Camelus dromedaries, Gene sequencing, Qalyubia Governorate.
Citation
Abdel-Aziz, A.R., El-Meghanawy, R.A., 2016. Molecular characterization of Hydatid Cyst from Egyptian one humped Camels (Camelus dromedaries). PSM Vet. Res., 01(1): 13-16.
Introduction
Livestock comprises of cattle, buffaloes, sheep, goats, camels, horses, asses and mules. There are a large number of parasites which infect the domesticated animals and are responsible for production loss (Iqbal et al., 2013; Iqbal et al., 2014; Muhammad et al., 2015; Omar et al., 2013). Several studies on the parasitic infections in domestic animals including sheep, camels, cattle, equines were done all over the world, and also was reported from one humped camels, especially in Egypt (Amer et al., 2015; Iqbal et al., 2014; Khalifa et al., 2014; Muhammad et al., 2015). Hydatid cyst infection involve the vital organs in the body of animals and human, so it is considered public health problem causing economic loss due to the condemnation of these organs at abattoirs. It is distributed worldwide especially the Sub- Saharan Africa (Wahlers et al., 2012). A number of studies were carried out depending on the mitochondrial DNA examination showed that Echinococcus granulosus complex having a high genetic diversity and variability and at least ten known strains (G1-G10) were identified as follows; G1 and G2 of them belonging to the ovine strain, G3 and G5 for bovine strains, G4 for the equine strain, G6 for camel strain, both G7 and G9 were for pig and the last two G8 and G10 strains for cervid strains (Cardona and Carmena, 2013).
The genetic studies of E. granulosus population has remarkable significance for epidemiological and control studies (Thompson et al., 2002). One previous study has explained the population structure of E. granulosus from Cairo, Egypt (Abdel-Aaty et al., 2012), molecular characterization of E. granulosus isolates was done from camels and humans by sequence and phylogenetic analyses in Qalyubia Governorate, Egypt (Omar et al., 2013).
The aim of this study was the investigation of the strains of E. granulosus present in camels in slaughter houses in Egypt using primers for mitochondrial and cytochrome oxidase gene in Qalyubia Governorate. It was considered the second attempt to identify the circulating genetic strains from one humped camels in this governorate.
Materials and Methods
Samples collection, and preparation
Fifty five hydatid cyst samples were taken only from the lung over a period of nearly one month from one humped camels from the Toukh’s abattoir (Northern to Cairo), Qalyubia Governorate, Egypt. Cysts were collected directly after evisceration of the slaughterd animals into flasks (thermo flasks) and transferred to the Molecular Biology unit, Assiut University for processing and molecular intervention. Hydatid fluids were collected into several test tubes after opening the cyst wall and then identified, centrifuged and supernatant excluded. The sediment (hydatid sand) which contains free scolices, brood capsules was collected into a clean sterile bottle having 70% ethyl alcohol for preservation and stored at -20°C (Osman et al., 2009).
DNA Extraction
Hydatid sand was washed by nucleic acid free water for removing ethyl alcohol preservative. DNA was extracted using the QIAamp tissue kit (QIAGEN, Germany) according to instructions on the kit. DNA was obtained by spinning in centrifuge at 12,000 RPM for 60 sec. at 37°C. 10 μl of the suspended DNA was used in the amplification reaction (McManus and Thompson, 2003).
Primers
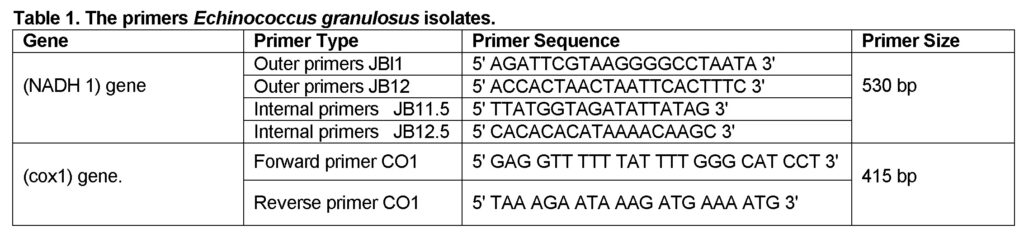
The primers for mitochondrial NADH dehydrogenase subunit 1 and cytochrome C oxidase subunit 1 gene for EG isolates (Bowles and McManus, 1993; Ibrahim et al., 2011) were used (Table 1).
PCR, Gene Sequence analysis and phylogeny
Polymerase chain reaction was done on the extracted DNA according to previous study (Nakao et al., 2007). The PCR product was purified by QIAquick PCR purification kit (QIAGEN) and then sent to Assiut university of Egypt the molecular biology unit, for the purpose of sequencing and then the sequence alignment was obtained by Local Alignment Search Tool (BLAS). It was compared to strains of known genotype from other countries of the world through NADH1 and cox1 subunit of genes. By using MEGA software version 5.0 (Tamura et al., 2011), the phylogenetic tree were then constructed as out groups (Ahmed et al., 2013).
Results and Discussion
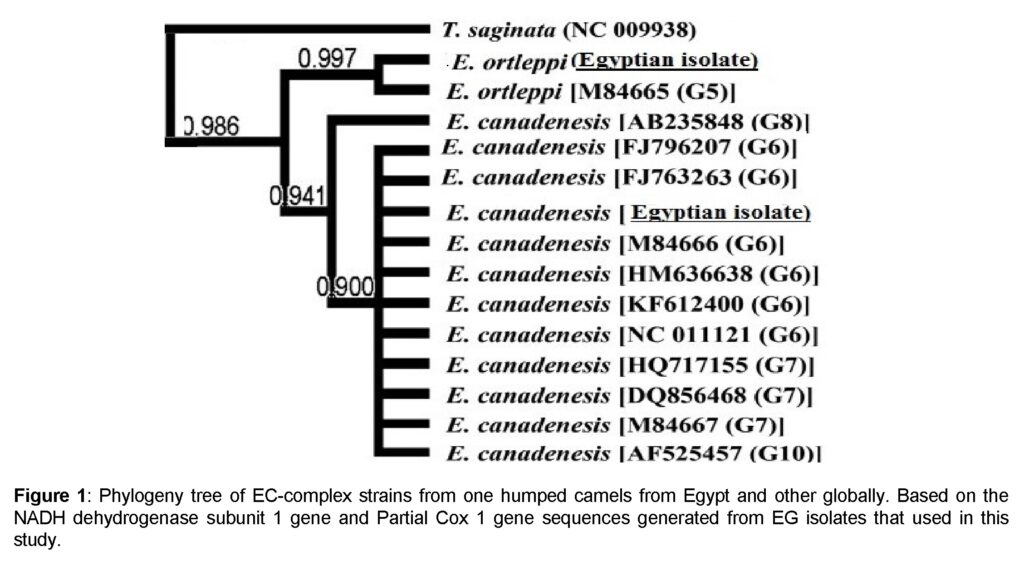
Results of the present study confirmed that the isolates under this study belong to the EG complex. It was found that 90.5% (49 out of 55 EG isolates) of samples had 100% homology with E. canadensis genotype 6 (G6) found mostly in camels genotype. However, only six EG isolates (9.5%) belonged to E. ortleppi which is the cattle genotype (G5) (Figure 1).
In Egypt, the camel genotype (G6) is the most common genotype of EG and the one humped camels (Camelus dromedaries) play a crucial role in the epidemiology (Omar et al., 2013). But from our point of view, this assumption needs more and more investigation. Some studies stated that this genotype is considered of low public health significance (Santivanez et al., 2013; Casulli et al., 2010). Recent works illustrated that the incidence of this genotype is higher than described in the previous studies (M’Rad et al., 2005). Several studies were organized on the biology and molecular characterization of Echinococus granulosus strains found in different parts of Egypt to identify the circumstances around the epidemiology of the hydatid infection in one humped camels. The first step in prevention and clearance of the infections from specific area is to identify the strain(s) incriminated in the dissemination of infection and determine its life cycle. In a previous study on hydatid cyst of lung from Qalyubia governorate, Egypt, different genotypes including G6 were identified by gene sequencing of the NADH 1 genes (Khalifa et al., 2014). However, no studies were done for determining the phylogeny of EG5 in Egypt. The circulation of major variant (G6) in Egyptian camels suggests that specific mechanisms are responsible for its persistence in this area. This is probably due to close relationships between dogs and camels in the study area (Dinkel et al., 2004).
The previous theory about the relation between dogs and abattoirs was confirmed. In poor communities as in this province, people perform the slaughtering process in the open space, so by this condition dogs can be easily fed on slaughtered animals viscera that could carry the hydatid cyst completing the life cycle to become adult inside intestine of dogs. This practice can easily maintain the camel strain (G6) inside the study area.
Further studies and EG strain genotyping and phylogeny can help for identifying the epidemiology of this isolate in Egypt. Availability of more data can help the public health authorities for management and prevention protocol for the infection. Our results coincided with the previous studies which were carried out for molecular identification of animal and human hydatid cysts. It showed that camel and human strains are the most similar pairs so camels are important hosts for the dissemination of human hydatidosis (Khalifa et al., 2014; Azab et al., 2004).
Conclusion
In conclusion, this study explored the presence of E. ortleppi the cattle genotype (G5) in the newly imported one humped camel from Sudan in Egypt for the first time at Qalyubia governorate, Egypt. It was the second study that revealed Echinococcus genotype of E. canadensis (G6) in camels. Therefore E. ortleppi strain must be considered during future studies especially the imported camels from Sudan that may provoke it to become endemic in Egypt.
Acknowledgement
Authors acknowledge the manager of Toukh abattoir for his assistance for sample collection. The authors are very grateful to Molecular Biology Unit team at Assiut University for technical assistance.
Authors’ Contributions
Rasha A. El Meghanawy collected samples, extracted the DNA and optimized PCR assay; analyzed the sequences; Amer R. Abdel Aziz designed the experiment and prepared the final manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Abdel-Aaty, H.E., Abdel-Hameed, D.M., Alam-Eldin, Y.H., El- Shenawy, S.F., Aminou, H.A., Makled, S.S., Darweesh, S.K., 2012. Molecular genotyping of E. granulosus in animal and human isolates from Egypt. Acta Tropica., 121(2): 125-128.
Ahmed, M.E., Eltom, K.H., Musa, N.O., Ali, I.A., Elamin, F.M., Grobusch, M.P., Aradaib, I.E., 2013. First Report on Circulation of Echinococcus ortleppi in the one Humped Camel (Camelus dromedaries), Sudan. BMC Vet. Res., 9: 127.
Amer, S., Helal, I.B., Kamau, E., Feng, Y., Xiao, L., 2015. Molecular Characterization of Echinococcus granulosus Sensu Lato from Farm Animals in Egypt. PLoS ONE, 10(3): e0118509. doi:10.1371/journal. pone.0118509
Azab, M.E., Bishara, S.A., Helmy, H., Oteifa, N.M., El-Hoseiny, L.M., Ramzy, R.M. and Ahmed, M.A., 2004. Molecular characterization of Egyptian human and animal Echinococcus granulosus isolates by RAPD-PCR technique. J. Egypt. Soc. Parasitol.,34(1): 83-96.
Bowles, J., McManus, D.P., 1993. NADH dehydrogenase 1 gene sequences compared for species and strains of the genus Echinococcus. Int. J. Parasitol., 23:969-972.
Cardona, G.A. Carmena, D., 2013. A review of the global prevalence, molecular epidemiology and economics of cystic echinococcosis in production animals. Vet. Parasitol.,192, 10-32.
Casulli, A., Zeyhle, E., Brunetti, E., Pozio, E., Meroni, V., Genco, F. and Filice, C., 2010. Molecular evidence of the camel strain (G6 genotype) of Echinococcus granulosus in humans from Turkana, Kenya. Trans. R. Soc. Trop. Med. Hyg.,104(1): 29-32.
Dinkel, A., Njoroge, E., Zimmerman, A., Walz, M., Zeyhle, E., Elmahdi, I., Mackenstedt, U., Romig, T., 2004. A PCR system for detection of species and genotypes of the Echinococcus granulosus complex, with reference to the epidemiological situation in eastern Africa. Int. J. Parasitol., 34:645-653.
Ibrahim, K., Romig, T., Peter, K., Omer, R.A., 2011. A molecular survey on cystic echinococcosis in Sinnar area, Blue Nile state (Sudan). Chinese Med. J., 124: 2829-2833.
Iqbal, M.N., Muhammad, A., Anjum, A.A., Shahzad, K.A., Ali, M.A., Ali, S., 2014. Epidemiology of Gigantocotyle explanatum in naturally infected buffaloes. Veterinaria, 1: 15-18.
Iqbal, M.N., Muhammad, A., Anjum, A.A., Shahzad, K.A., Ali, M.A., Ali, S., 2014. Prevalence of Gastrothylax crumenifer in the gastrointestine of Bubalus bubalis. Veterinaria, 1: 28-31.
Iqbal, M.N., Shahzad, K.A., Muhammad, A., 2013. Identification and prevalence of Paraphistomum cervi in naturally infected water buffaloes of central Punjab, Pakistan. Veterinaria, 1: 9-12.
Khalifa, N.O., Khater, H.F., Fahmy, H.A., Radwan, M.E.I., Afify, J.S.A., 2014. Genotyping and Phylogenetic Analysis of Cystic Echinococcosis Isolated from Camels and Humans in Egypt. Am. J. Epidemiol. Infect. Dis., 2(3): 74-82. doi: 10.12691/ajeid-2-3-2.
McManus, D.P., Thompson, R.C.A., 2003. Molecular epidemiology of cystic echinococcosis. Parasitol., 127: 37-51.
M’Rad, S., Filisetti, D., Oudni, M., Mekki, M., Belguith, M., Nouri, A., Sayadi, T., Lahmar, S., Candolfi, E., Azaiez, R, Mezhoud, H. and Babba, H. (2005). Molecular evidence of ovine (G1) and camel (G6) strains of Echinococcus granulosus in Tunisia and putative role of cattle in human contamination. Vet. Parasitol.,129: 267-272.
Muhammad, A., Ahmed, H., Iqbal, M.N., Qayyum, M., 2015. Detection of multiple anthelmintic resistance of Haemonchus contortus and Teladorsagia circumcincta in sheep and goats of Northern Punjab, Pakistan. Kafkas. Univ. Vet. Fak. Derg., 21 (3): 389-395.
Muhammad, A., Shah, S.I., Iqbal, M.N., Ali, S., Irfan, M., Ahmad, A., Qayyum, M., 2015. Prevalence of Gigantocotyle explanatum in buffaloes slaughtered at Sihala Abattoir, Rawalpindi. Punjab Univ. J. Zool., 30(1): 11-14.
Nakao, M., McManus, D.P., Schantz, P.M., Craig, P.S., Ito, A., 2007. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitol., 134:713-722.
Omar, M., Sultan, K., Haridy, M., Omran, A., 2013. Prevalence of cystic Echinococcosis in slaughtered ruminants in different Abattoirs, upper Egypt. Am. J. Anim. Vet. Sci.,8(3): 117-121. doi:10.3844/ajavssp.2013.117.121
Osman, A.M., Aradaib, I.E., Ashmaig, A.K. and Gameel, A.A., 2009. Detection and differentiation of Echinococcus granulosus– complex using a simple PCR based assay. Int. J. Trop. Med.,4(1): 21-26.
Santivanez, S.J., Gutierrez, A.M., Rosenzvit, M.C., Muzulin, P.M., Rodriguez, M.L., Vasquez, J.C., Rodriguez, S., Gonzalez, A.E., Gilman, R.H., Garcia, H.H., The Cysticercosis Working Group in Peru, 2008. Human hydatid disease in Peru is basically restricted to Echinococcus granulosus Genotype G1. Am. J. Trop. Med. Hyg.,79 (1):89-92.
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M., Kumar, S., 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol., 28(10): 2731-2739.
Thompson, R.C., McManus, D.P., 2002. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol., 18(10): 452-457.
Wahlers, K., Menezes, C.N., Wong, M.L., Zeyhle, E., Ahmed, M.E., Ocaido, M., Stijnis, C., Romig, T., Kern, P., Grobusch, M.P., 2012. Cystic echinococcosisin sub-Saharan Africa. Lancet Infect. Dis., 12: 871-880.