Effect of Sodium Selenite on Kidney Function Test of Rabbits
1Department of Anatomy and Histology, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan
2The School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China
3Pakistan Science Mission (PSM), Noor Kot 51770, Pakistan.
4Department of Zoology, Lahore College for Women University, Lahore, 54000, Pakistan.
5Department of Zoology, Govt. Post Graduate Islamia College (W) Cooper Road, Lahore54000, Pakistan.
*Corresponding author:
Asfa Ashraf;Email:
sundausnaeem@yahoo.comViews 3569
Abstract
This study was conducted to evaluate the effect of Sodium Selenite at different dosage levels mixed with water in rabbit. Fifteen rabbits (120 day old) were purchased from Tolinton market, Lahore. They were divided into three groups having three replicas (5 rabbits each). One group was kept as un-medicated control A. While the other Groups such as B and C were medicated with sodium selenite in a single dose at the rate of 2 and 3 mg/kg of body mass mixed in water for 20 days consecutively. Control diet Group “A” received basal diet without any supplementation. Blood samples were collected on 1st, 7th, 14th, 21st and 28th day of dietry treatment from each group. Various serological parameters of kidney functioning enzymes (RFT) such as Creatinine and Urea were determined by using Semi automated Chemistry Analyzer. A significant increase in Creatinine and Urea levels (p<0.05) in rabbits of various treated groups was recorded after applying statistical ANOVA test. The results revealed that Sodium selenite alters the serum creatinine and urea levels by affecting the kidney and cause renal toxicity in rabbits.
Keywords
Rabbits, Serological parameters, Kidney function enzymes test, Creatinine test, Urea test.
Citation
Alam, S., Masood, S., Iqbal, M.N., Ashraf, A., Yunus, F.N., Xiao, S., Ahsan, M., 2016. Effect of Sodium Selenite on Kidney Function Test of Rabbits. PSM Vet. Res., 01(1): 17-21.
Introduction
Essential minerals have a metabolic role in the body and are divided into two groups such as the major elements (cadmium, phosphorous, potassium, Sodium, chlorine and magnesium) and trace minerals (iron, iodine, copper, manganese, selenium and zinc) (Donald et al., 1987). Selenium is a trace element and act as cofactor in enzymatic functions and other metabolic processes (Rooney, 2007) and plays a key role in functions of immune system, regulation of thyroid hormone metabolism and enzymatic antioxidant defenses (Iglesias et al., 2013). Selenium is an important nutrient in the nutrition of organisms. It is nonmetallic element that causes toxicity in different types of animals when it is ingested by animals from various sources such as plants growing in soils (Franchini and Bertuzzi, 1991).
Selenium is washed out from the soil by the water; due to this reason the plants are able to take selenium form water along with the soil. The concentrations of selenium in plants vary; those plants are able to accumulate selenium against a concentration gradient are known as selenium accumulators, which is responsible for the transport of selenium to the people at the end of food chain (Dumont et al., 2006). Selenium passes placental barriers as well as through mother milk to suckling due to transportation in the organism to various tissues and cells, after ingestion it is reached to the soil through feces (Allen and Miller, 1981). Soil bacteria are responsible for conversion of selenium to selenite. Interest is increasing in the selenium compound due to their biological and toxicological importance and in particular, because of their anticancer activities (Combs, 2001).
The high concentration of selenium causes toxic effects in organisms. Chronic selenium poisoning, introduce selenosis in humans and produces alteration in the liver in China (Gailer et al., 2000). Sensitivity to viral infections is increased by the Selenium insufficiency and antioxidant status. Kashin-Beck disease (KBD) is the syndrome caused by lack of selenium together with the iodine deficiency and causes severe disorders such as atrophy, degeneration and necrosis of cartilage tissues (Hol et al., 2001). The oxidative stress resulted from selenium deficiency results in the development of abnormal oxidation reduction system in organs and cells (Bordoni et al., 2007).
Toxic levels of Se (10-20 ppm) are more than 100-fold higher the nutritional requirements. Usually Se doses lower than 3-5 ppm in animal feed are not associated with toxicity (Surai et al., 2002a). The consumption of higher levels of Se can cause selenosis, characterized by hair loss, gastrointestinal upsets, white blotchy nails, fatigue, irritability, and mild nerve damage in both humans and animals. Sodium selenite is toxic than less soluble elemental selenium, selenium sulfide and selenium disulfide (Koller et al., 1986).
The purpose of this study was to notice the toxic effect of sodium selenite on kidney function test in rabbit.
Materials and Methods
Experimental Animals
The experiment was conducted on 120 day old (two months) clinically healthy fifteen rabbits with a mean weight of 1kg. These rabbits were purchased from the Tolinton market, Lahore, Pakistan. The rabbits were divided into three groups. Each group contains 5 animals. One of these groups (A) was kept as a control group. Other two (B&C) were served as experimental groups. Rabbits of group B and Group C were identified from each other by ribbon tags of orange and green color respectively.
Acclimatization
The rabbits were allowed to acclimatize to laboratory condition in well- ventilated cage for 5 days. They had access to feed and water all the time. They were kept under standard conditions of temperature (37 0C) and humidity (40-50%). Care of the animals was taken as per guidelines of European Communities Council Directive of 24 November 1986 (86/609/EEC) and the study was approved by Institutional Animal Ethics Committee.
Toxicant
Selenite was used in the form of Sodium selenite. This toxicant was mixed with water in varying amount. Sodium selenite was purchased from BDH chemicals Ltd.
Dietary plan
Control group (A) was fed with simple diet mixed with water. Experimental diets mixed with water containing 2mg/kg and 3mg/kg of Sodium selenite were given to group B and C rabbits respectively.
Parameters studied
Following parameters were studied
Body weight
Body weight was determined by an electric balance (SHIMADZU BX-3000 Japan). The rabbits were weighted on very first day of experiment to have initial body weight and then on weekly bases to calculate the body weight changes till the termination of the experiment. The weight changing was calculated by previous body weight from new body weight.
Blood collection
The blood samples were collected every 7th day (one week) from cephalous vein of rabbits with sterile disposable syringe after rubbing vein with xylene, so that the veins become swollen. The needle of 24 gauges was used for sucking the blood, which was inserted at the angle of 45. 2ml blood (each time) was collected in test tubes for serological studies (RFT).
Serum separation
The serum was isolated according to the procedure reported in manual of basic Techniques for healthy laboratory (WHO-Geneva, 1980).
- The sampling tubes containing blood were allowed to clot at room temperature for about 2-3 hours.
- The clotted blood was then centrifuged at 4000 rpm for about 15 minutes.
- After centrifugation the cellular portion of blood along with fibrinogen was accumulated in bottom of centrifuge tube and light straw color serum was then separated.
- The serum was then transferred to sterile eppendroff tubes with the help of pasture’s pipette.
- Finally eppendroff tubes were capped and labeled for storage.
- The serum samples of each rabbit was collected and stored separately.
Storage of serum
The labeled sampling eppendroff tubes containing serum were stored in the freezer compartment of the refrigerator at -8oC to -10oC until they were subjected to the analysis. Serum once separated could be stored for fairly long time by freezing.
Kidney Function Test (RFT)
The levels of serum creatinine and urea were determined based on the following the principles.
Creatinine
The end product of reaction of creatinine with alkaline picrate is an orange yellow color (Jaffe’s reaction). There is direct proportion between absorbance of orange yellow color and amount of creatinine in the sample. The absorbance is measured at 510nm (Patil et al., 2014). The amount of creatinine is determined in mg ∕ dl.
Urea
The end products of hydrolysis of urea in the presence of water and urease are ammonia and carbon dioxide. Ammonia reacts with ketoglutarate in the presence of glutamate dehydrogenase (GLDH) and reduced nicotinamide adenide dinucleotide (NADH) to produce L-glutamate. Adenosine diphosphate (ADP) is used as an activator and stabilizer of GLDH. The results of reaction are determined by measuring the decrease in absorbance at 340 nm, as NADH is oxidized to NAD. The value is expressed in mg/dl (Patil et al., 2014).
Statistical analysis
Statistical analysis was performed through general linear models. Univeriate ANOVA was applied for the parameters (Scheffe, 1959) at 95% confidence interval. The significant values were expressed by asterisk (p < 0.05). The data was expressed as mean ±S.D.
Results
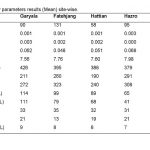
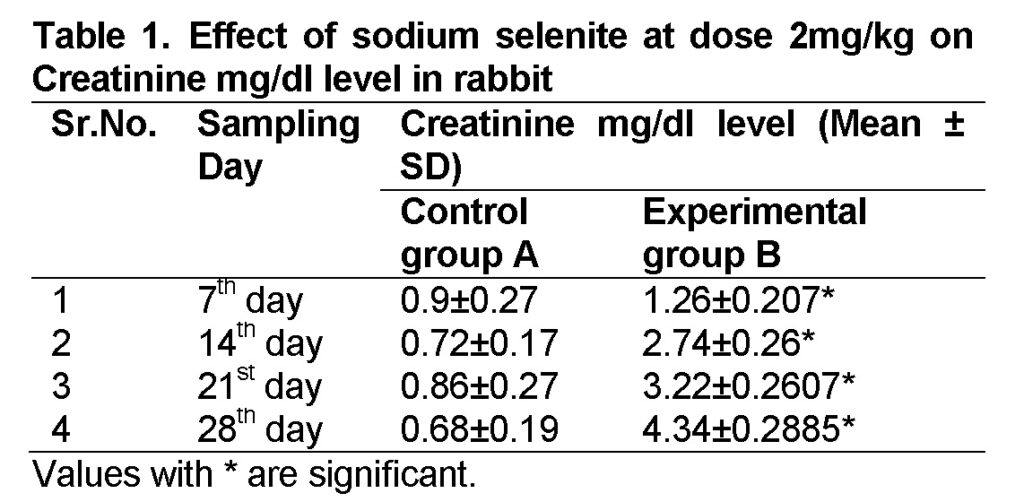
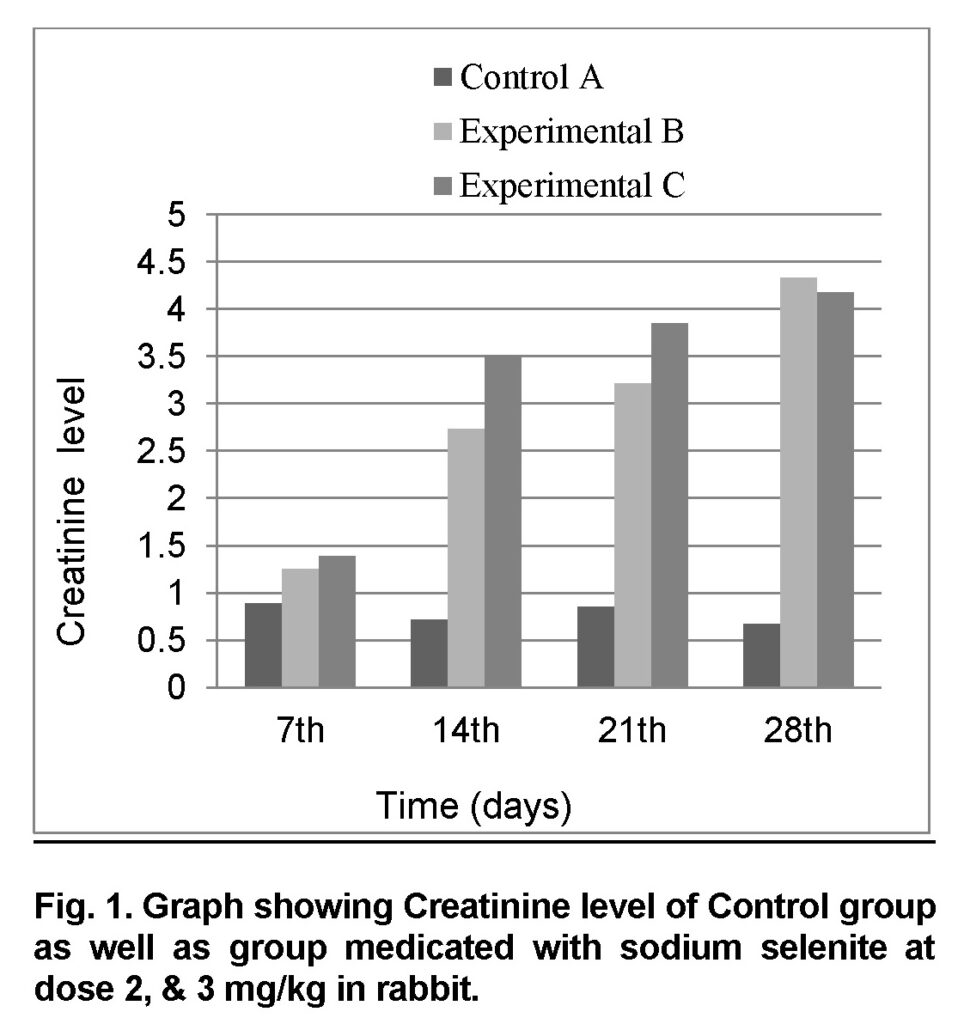
Influence of Sodium selenite at dose of 2mg/kg in group “B” was analyzed. The mean values of Creatinine in experimental group B on 7st, 14th, 21th, and 28th days were 1.26 ± 0.207, 2.74 ± 0.26, 3.22 ± 0.2607 and 4.34 ± 0.2885 mg/dl (Table 1). The level of Creatinine increased in group “B” as compared to control at the end of 28th day of the experiment (Figure 1). The value of F was 10.746 showed highly significant results. The level of significance was 0.017 that is less than 0.05.
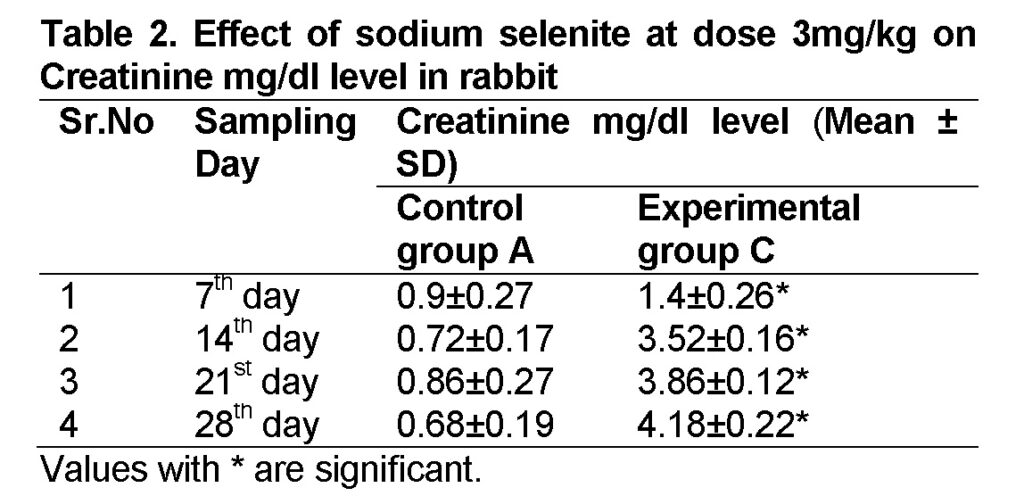
Influence of Sodium selenite at dose of 3mg/kg in group “C” was analyzed. The mean values of Creatinine level in experimental group C on 7st, 14th, 21th, and 28th days were 1.4 ± 0.26, 3.52 ± 0.16, 3.86 ± 0.12 and 4.18 ± 0.22 mg/dl (Table 2). The level of Creatinine was increased in group “C” as compared to control at the end of 28th day of the experiment (Figure 1). The value of F was 24.749 showed highly significant results. The level of significance was 0.003 that is less than 0.05.
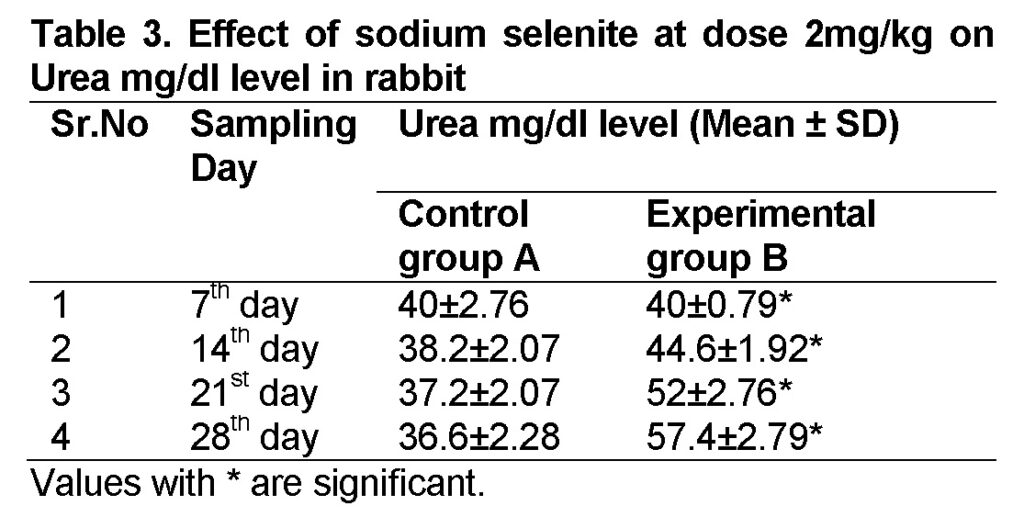
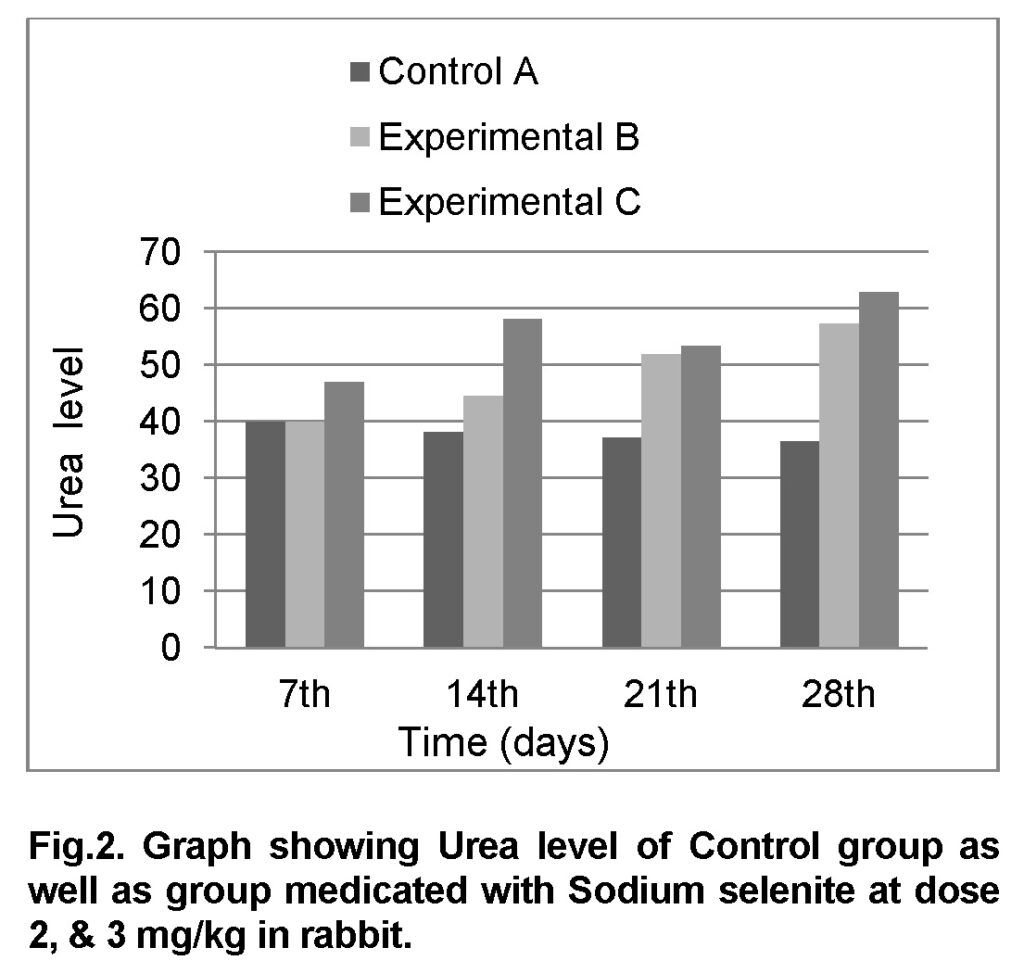
Influence of Sodium selenite at dose of 2mg/kg in group “B” was analyzed. The mean values of urea level in experimental group B on 7st, 14th, 21th, and 28th days were 40 ± 0.79, 44.6 ± 1.92, 52 ± 2.76 and 57.4 ± 2.79 mg/dl (Table 3). The level of urea was increased in group “B” as compared to control at the end of 28th day of the experiment (Figure 2). The value of F was 7.130 showed highly significant results. The value of significance was 0.037, is less than 0.05.
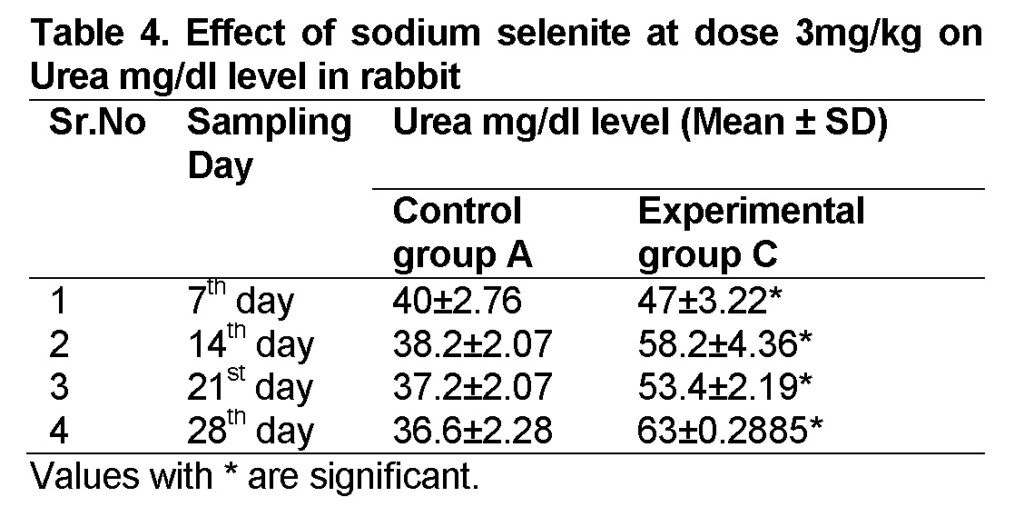
Influence of Sodium selenite at dose of 3mg/kg in group “C” was analyzed. The values of urea level in experimental group C on 7st, 14th, 21th, and 28th days were 47 ± 3.22, 58.2 ± 4.36, 53.4 ± 2.19 and 63 ± 2.42 mg/dl (Table 4). The level of urea was increased in group “C” as compared to control at the end of 28th day of the experiment (Figure 2). The value of F was 24.749 showed highly significant results. The value of significance was 0.003, is less than 0.05.
Discussion
In poultry and livestock Sodium selenite feed are used to support growth, and its forms are present in the waste chemical disposal sites which are indirectly reached to the ground or drinking water.
Serum concentrations of urea nitrogen and creatinine are traditional screening tests used to evaluate renal function. Our results showed that higher level of sodium selenite in the diet of experimental rabbits is responsible for the increase in creatinine in blood serum. The highest amount of creatinine was found at 28th day. The presence of higher amount of creatinine in blood serum is responsible for nephrotoxicity. Previous studies also demonstrated raised amount of serum creatinine after feeding rabbits with selium supplemented forage. The serum creatinine showed significant difference (P<0.05) among treatment (Saleh et al., 2015).
Creatinine test has advantages over urea as a renal function test because it is inactive metabolically. Creatinine is derived entirely from endogenous metabolism. Its excretion is independent of diet (Krishnaswamy and Lukose, 2015). When renal function fails, the serum Creatinine rises. The Creatinine clearance is by far the most sensitive regular clinical test of renal functioning available today. Normal values of Creatinine vary depending on the method and specimen used (Rule et al., 2004). Creatinine ratio can be used to draw additional clinical data about the status of renal function (Levey et al., 2006). In present study the level of Creatinine concentration was significantly higher in case of Group B and C as compared to Group A indicating renal toxicity. The results of previous studies suggested that selenium was more toxic to female rats than to males (Fitzhugh et al., 1944). Schroeder (1967) observed a higher number of deaths in male Long-Evans rats than in females receiving 2 ppm selenium as sodium selenite in the drinking water. Like kidney function tests, variable values of serum parameters for liver function tests are responsible for liver disorders in humans (Muhammad et al., 2013; Toor et al., 2016).
Our results showed that higher level of sodium selenite in the diet of experimental rabbits is responsible for the increase in urea in blood serum. The highest amount of urea was found at 28th day. The presence of higher amount of urea in blood serum is responsible for nephrotoxicity. Previous studies also demonstrated raised amount of serum urea after feeding rabbits with selium supplemented forage (Saleh et al., 2015). The serum urea values were within the normal range reported previously (Njidda and Isidahomen, 2011; Njidda and Isidahomen, 2010) who fed sesame seed meal and grasshopper meal to rabbit. Duncan and Prasse (1986) reported lower serum urea values that are against our findings. In many renal diseases, the glomerular filtration rates of two kidney falls considerably below normal resulting in decreased excretion of urea (Guyton 1991). However, the body continues to form large quantities of urea which means the urea will progressively collect in the body fluids until its plasma concentration rises very high. Higher concentrations of urea and creatinine in blood are responsible for renal toxicity.
Conclusion
Observation of current study describes that sodium selenite changes the level of the serum Creatinnine and Urea in Rabbits (especially in the case when affected by the liver). The toxicity is higher when administered by the high dose of sodium selenite in the diet as compared to the low dose.
Acknowledgement
The authors are grateful to Dr. Saima Masood, Department of Anatomy and Histology, University of Veterinary and Animal Sciences, Lahore, Pakistan for her kind guidance.
Conflict of Interest
The authors declare that they have no competing interests.
References
Allen, J.C., Miller, W.J., 1981.Transfer of selenium from blood to milk in goats and noninterference of copper with selenium metabolism. J. Dairy Sci., 64: 814-821.
Bordoni A.,Danesi F., Malaguti M, Nunzio M.D., Pasqui F., Maranesi M., and Biagi P.L.., Dietary Selenium for counteraction of oxidative damage fortified foods or supplementation . Br. J. Nutr. 2007, 26ː page 1-7.
Combs, G.F., 2001, Selenium in global food systems. Br. J. Nutr.,85:517-547.
Donald, M.C., Edward, P., Green, R.A., Halgh., 1987. ‟Animal Nutrition” 4th Ed, English Language Book Society Longman.
Dumont, E., Vanhaecke, F., Cornelis, R., 2006. Selenium speciation from food source to metabolites: a critical review. Anal. Bioanal. Chem., 385: 1304-1323.
Duncan, J.R., Prasse, K.W., 1986. Veterinary Laboratory Medicine, 2nd ed., Iowa State University Press.
Fairbrother, A., Fowles, J., 1990. Subchronic effects of sodium selenite and selenomethionine on several immune-functions in mallards. Arch. Environ. Contam. Toxicol., 19: 836-844.
Fitzhugh, O.G., Nelson, A.A., and bliss, C.I., 1944. The chronic oral toxicity of selenium. J. Pharmacol. Exp. Ther., 80: 289-299.
Gailer, J., George, G.N., Pickering, I.J., Madden, S., Prince, R.C., Yu, E.Y., Denton, M.B., Younis, H.S., Aposhian, H.V., 2000. Structural basis of the antagonism between inorganic mercury and selenium in mammals. Chem. Res. Toxicol., 13(11):1135-42.
Guyton, A.C., 1991. Blood pressure control-special role of the kidneys and body fluids. Sci., 252: 1813-1816.
Hol, P.J., Vamnes, J.S., Gjerdet, N.R., Eide, R., Isrenn, R., 2001. Dental amalgam and selenium in blood. Environ. Res., 87: 141-146.
Iglesias, P., Selgas, R., Romero, S., Diez, J.J., 2013. Selenium and kidney disease. J. Nephrol., 26(2): 266-72.
Koller, L.D., Exon, J.H., 1986. The two faces of selenium-deficiency and toxicity are similar in animals and man. Can. J. Vet. Res., 50: 297-306.
Krishnaswamy, R., Lukose, S., 2015. Evaluation of the three methods available for the estimation of creatinine Clearance. Int. J. Clin. Biochem. Res., 2(2): 83-88.
Levey, A.S., Coresh, J., Greene, T., Stevens, L.A., Zhang, Y.L., Hendriksen, S., Kusek, J.W., Van., Lente, F., 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med., 145: 247-254.
Muhammad, A., Farooq, M.U., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., 2013. Prevalence of diabetes mellitus type II in patients with hepatitis C and association with other risk factors. Punjab Univ. J. Zool., 28 (2): 69-75.
Njidda, A.A., Isidahomen, C.E., 2010. Hematology, Blood chemistry and carcass characteristics of growing rabbits fed grasshopper meal. Pak. Vet. J., 30(1): 7-12.
Njidda, A.A., Isidahomen, C.E., 2011. Hematological parameters and carcass characteristics of weanling rabbits fed sesame seed meal (Sesamumindicum) in a semi-arid region. Pak. Vet. J., 31(1): 35-39.
Patil, A.N., Arora, T., Desai, A., Tripathi, C.D., 2014. Comparison of the Species-Sensitive Effects of Different Dosages of Calcium and Verapamil on Gentamicin-Induced Nephrotoxicity in Rats and Rabbits. Toxicol. Int., 21(3): 225-231.
Rooney, J.P., 2007. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicol., 234: 145-156.
Rule, A.D., Larson, T.S., Bergstralh, E.J., Slezak, J.M., Jacobsen, S.J., Cosio F.G., 2004. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann. Intern. Med., 141:929-937.
Saleh, J.L., Njidda, A.A., Adeniji, A.A., Lawan, G.B., 2015. Haematological and Biochemical Indices of Rabbits Fed Graded Levels Browse Forage (Balanites aegyptiaca) in Semi-Arid Environment. Global J. Sci. Front. Res., 14(2): 43-48.
Scheffé, H. (1959), The Analysis of Variance, New York: John Wiley & Sons.
Schroeder, H.A. and Mitchener, M., 1971. Selenium and tellurium in rats: effect on growth, survival and tumors. J. Nutr., 101: 1531-1540.
Surai, P.F., 2002a. Selenium in poultry nutrition 1. Antioxidant properties, deficiency and toxicity.Worlds Poult.Sci. J., 58, 333-47.
Toor, S., Toor, S., Ashraf, A., Alam, S., Anwaar, S., Saddiqa, A., Ali, S., Muhammad, A., Toor, S., Akhter, S., 2016. Prevalence of Liver disorders in Islamabad City. PSM Biol. Res., 01(1): 31-33.
WHO-Geneva.1980. Manual of Basic Techniques for a Health Laboratory. E.R.I.C, ISBN: ISBN-92-4-154145-8.