Antibacterial Activities of Methanolic Extracts of Datura inoxia
1Key Laboratory of Microbial Diversity in Southwest China, Ministry of Education, Yunnan Institute of Microbiology, Yunnan University, Kunming, 650091, PR China.
2Department of Microbiology Kohat University of Science & Technology
3The School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China.
4Pakistan Science Mission (PSM), Noor Kot 51770, Pakistan.
5Department of Zoology, Lahore College for Women University, Lahore 54000, Pakistan.
Corresponding author:
Firasat Hussain;Email:
hussain_great19@yahoo.comViews 2642
Abstract
Indigenous plants are valuable and traditionally used against various ailments. These plants are used for the synthesis of numerous drugs. Present work was based on the research conducted on methanolic crude plant extracts of leaves, stem, root and seeds of Datura inoxia. Plant was screened to investigate the antibacterial activities. Antibacterial screening was performed against Escherichia Coli, Pseudomonas aeruginosa, Staphylococcus aureus, Klebsiella pneumoniae, Proteus spp., Streptococcus pneumoniae and Salmonella typhi by following the agar well diffusion method. Considerable biological activities were exhibited by each methanolic extracts of plant ranging diameter of inhibition zones from 6 mm to 24 mm. It is concluded that plant extracts should be preferred for treatment of various infectious diseases.
Keywords
Drugs, antibacterial, biological activities, agar well diffusion.
Citation
Hussain, F., Kalim, M., Ali, H., Ali, T., Khan, M., Xiao, S., Iqbal, M.N., Ashraf, A., 2016. Antibacterial Activities of Methanolic Extracts of Datura inoxia. PSM Microbiol., 01(1): 33-35.
Introduction
In modern and traditional medicines, medicinal plants constitute an effective source. Plants act as a biosynthetic laboratory for its chemical compounds like alkaloids, phenolics, turpenoids and glycosides. Natural products have played an important role in treatment and prevention of human diseases (Newman et al., 2000; Chin et al., 2006; Kaushik et al., 2008). There are reports of about 85,000 valuable medicinal plant species world-wide (Deviet al., 2009; Liu and wang, 2008). The novel drugs were developed by the extraction of biologically active compounds from plants which were screened on the basis of medicinal uses or bioactivity (Hunter, 2001). Many researchers have inspected long-established uses of medicinal plants, but only a few studies have confirmed antimicrobial properties of these plants (Bhattarai et al., 2008a; Shakya et al., 2008). About 80% of population in developing countries uses medicinal plants as traditional health remedies as they lack access to pharmaceutical drugs (Magrani et al., 2005).
Pakistan has unique biodiversity on earth comprising of different climatic zones with a variety of plant species. There are estimates of existence of about 6000 plant species of medicinal plants in Pakistan. Natural products have been used as good sources of pharmacologically active compounds (Ahmed, 2015). Plants belonging to family Solanaceae are distributed worldwide, which includes 85 genera and about 2,800 species in the world. There are approximately 25 different species of Datura throughout the world, they are often called as Jimson weed or ‘Thornapple’. The name Datura is derived from the early Sanskrit Dustura (Mann, 1996) or dahatura. Datura has numerous common names like deadly nightshade, Thorn apple, Stink weed, Jimson weed, Devil’s apple and angel’s trumpet (Heiser, 1969).
The whole plant is antiseptic, narcotic, sedative and is useful for asthma (Bhattacharjee and Supriya Kumar, 1998). Typical height of this plant is from 0.6 to 1.5 meters. The leaves and stems are protected by soft and short grayish hairs, thus whole plant appears grayish. The trumpet-shaped, white flowers are 12-19 cm long. The fruit of Datura plant is an egg- shaped spiny capsule which is about 5 cm in diameter. Datura seeds have shown 90 % germination rate even after 39 years of storage (Heiser, 1969). The germination time for seeds is usually 3 – 6 weeks at 15 °C.
The objective of the present study was to investigate the antibacterial activities of of methanolic extracts of Datura inoxia.
Materials and Methods
Collection of Plant Material
The fresh matured plant of Datura inoxia was collected during summer season from a natural population of University of Peshawar. The plant species of Datura inoxia were identified with reference to standard morphological characteristic features following the flora of Pakistan.
Test Microorganisms
The methanol extracts of Datura innoxia were tested against the following seven bacterial strains:Escherichia Coli, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus spp., Klebsiella pneumoniae, Salmonella typhi and Streptococcus pneumoniae.These bacterial strains were retrieved from stock culture of Microbiological Research Laboratory, Centre of Biotechnology and Microbiology, University of Peshawar.
On nutrient agar plate bacterial species were maintained and thus recovered for testing by sub culturing in nutrient broth for 24 hrs. Stock cultures were stored at 4°C. For growth and maintenance of bacterial cultures Nutrient agar, Nutrient broth, Dextrose Agar), and MH (Mueller Hinton Agar) were used.
Extract Preparation
Fresh parts of plants i.e: leaves, stem, root, and seeds were collected from Datura inoxia plant and washed under tap water, dried in shade and used for extraction. These leaves, stem, root and seeds were retained in dark room for drying at 37 °C for 15-20 days. These air dried parts of plant were placed in methanol for the extraction of active compounds. These were kept at room temperature for 15 days, observed and jiggle every day. Then the extracts were filtered. The soluble extracts were poured in isolated flasks. The solvents were evaporated for 1 hour at 50 °C by rotatory evaporator. Finally 5 gm crude extract of leaves and seeds were obtained separately. Similarly 8 gm extracts from stem and roots were isolated. For further use, the extracts were stored at 4 °C.
Antibacterial Activity Testing
All the processes were carried out in sterilized environment. Nutrient agar slants were used for stock culture at 4 °C. Stock culture of bacteria was transfered to tubes containing 5 ml nutrient broth. These were poured on MH plates, rotated uniformly and kept for 30 minutes. So that microorganisms adheres on solid surface. Sterilized cork borer having 4 mm diameter was used for wells making. 2 mg of extracts were dissolved in 1 ml DMSO. Agar well diffusion method (Perez et al., 1990)was employed. 10 µl solutions were pipette out and poured in wells to check the antibacterial activities of each parts of Datura inoxia separately. Then plates were placed in an incubator at 37°C for 24 hrs to allow the bacterial growth and check out the active components of Datura inoxia against bacterial species. DMSO was used as negative control and 2 mg of erythromycin and streptomycin were dissolved in standard sterile distal water, considered as positive control.
Results and Discussion
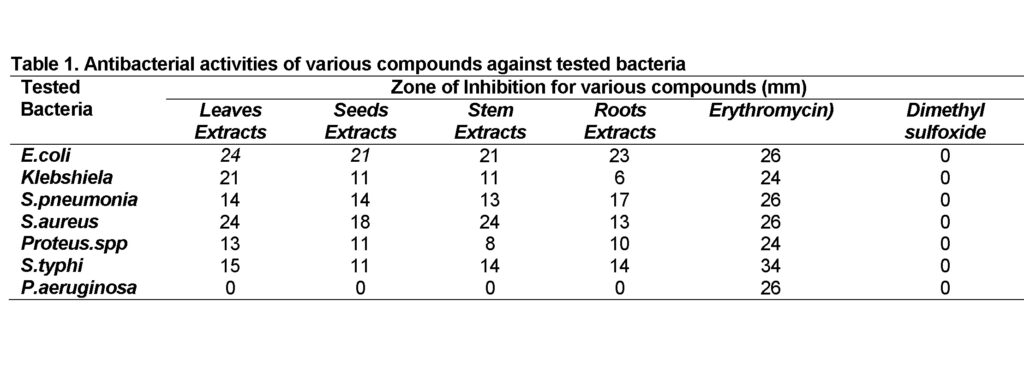
The systematic screening of extracts from antimicrobial plants represents a continuous effort to find new compounds which are potentially active against multiresistant pathogenic bacteria and fungi. Methanol extracts from the leaves, stem, roots and seeds of Datura inoxia at 2 mg concentration were isolated and showed inhibition by well diffusion method ranging from 6 mm to 24 mm as shown in table 1. Escherichia Coli, Salmonella typhi, Klebsiella pneumoniae, Proteus spp., Streptococcus pneumoniae and Staphylococcus aureus were more affected. The Pseudomonas aeruginosa showed resistance. Our results of antibacterial activity by methanolic extracts of Datura inoxia are in accordance to previous findings (Joshi and Kaur, 2013; Mathur et al., 2013).
Natural products are continuously provided by plants which find extensive application in curing diseases and fulfilling various other everyday needs. There is great increase in multiple antimicrobial resistances in human pathogens by the use of commercial antimicrobial drugs. Nowadays it is a serious threat that some antibiotics show undesirable side effects and are responsible of emergence of infections (Marchese and Schito, 2000). This creates a great trouble for scientists and forced them to search for new antimicrobial substances from different sources like the medicinal plants. The analysis and screening of plant products and plant extracts for antimicrobial activities have demonstrated that higher plants serve as an important source of novel antibiotic protypes (Meurer-Grimesa et al., 1996). It has been inherited to use the medicinal plants as an important part of the health care system. About 20 % of the plants present on the earth have been submitted to test the pharmacological or biological activities (Suffredini et al., 2004). Plant derivatives like honey have shown antibacterial activity against B. alvei, B. polymyxa, B. subtilis and S. aureus (Iqbal et al., 2015).
Conclusion
The methanol extracts of leaves, stem, roots and seeds of Datura inoxia showed activities against tested bacterial species. It was concluded that medicinal plants have huge contribution to the traditional and western medicine by providing ingredients for drug or being used in the drug discoveries.
Acknowledgement
This research was supported by Department of Microbiology, Kohat University of Science and Technology and also appreciates the scientific assistance from Microbiological Research Laboratory, Centre of Biotechnology and Microbiology, University of Peshawar.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Ahmed, M., 2015. Some medicinal plant resources and traditional uses in Pakistan. J. Plant Breed. Crop Sci., 7(5): 158-162.
Bhattarai, S., Chaudhary, R.P., Taylor, R.S.L., 2008a. Screening of selected ethno medicinal plants of Manang district, Central Nepal for antibacterial activity. Ethnobot., 20: 9-15.
Bhattacharjee and Supriya Kumar, 1998. “Hand book of Medicinal plants”. Pointer Publishers, Jaipur, 124.
Chin, Y.W., Balunas, M.J., Chai, H.B., Kinghorn, A.D., 2006. Drug discovery from natural sources. AAPS J., 8(2):E239-E253.
Devi, K., Karthikai, G., Thirumaran, G., Arumugam, R., Anantharaman, P., 2009. Antibacterial Activity of Selected Medicinal Plants from Parangipettai Coastal Regions; Southeast Coast of India. World Appl. Sci. J., 7(9): 1212-1215.
Heiser, C.B., 1969. Nightshades: The Paradoxical Plants. San Francisco: W. H. Freeman. p 143.
Hunter, D., 2001. Life in the fast lane: high throughput chemistry for lead generation and optimization. J. Cell. Biochem., 84(Suppl. 37): 22-27.
Iqbal, M.N., Anjum, A.A., Ali, M.A., Hussain, F., Ali, S., Muhammad, A., Irfan, M., Ahmad, A., Shabbir, A., 2015. Assessment of microbial load of un-pasteurized fruit juices and in vitro antibacterial potential of Honey against bacterial isolates. Open Microbiol. J., 9: 26-32. DOI: 10.2174/1874285801509010026
Joshi, M., Kaur, S., 2013. In vitro evaluation of antibacterial activity and phytochemical evaluation of Datua inoxia leaves. Asian J. Pharm. Clin. Res., 6(5): 25-28.
Kaushik, P., Chauhan, A., Chauhan, G., Goyal, P., 2008. Evaluation of Nostoc commune for potential antibacterial activity and UV-HPLC analysis of methanol extract. Internet J. Microbiol., 5(1): 1-5.
Liu, Y., Wang, M.W., 2008. Botanical drugs: Challenges and opportunities contribution to Linnaeus memorial symposium 2007. Life Sc., 82(9-10): 445-449.
Magrani, M., Zeegwah, N.A., Michel, J.B., Eddouks, M., 2005. Antihypertensive effect of Lepidium sativum in spontaneously hypertensive rats. J. Ethnopharmacol., 100(1-2): 193-197.
Mann, J.. 1996, “Murder, magic and medicine” Oxford university Press Oxford, 82-84.
Marchese, A., Schito, G.C., 2000. Resistance patterns of lower respiratory tract pathogens in Europe, Int. J. Antimicrob. Agents, 16(Suppl. 1): 25 – 29.
Mathur, P., Singh, A., Shrivastava, V., Singh, D., Mishra, Y., 2013. Antimicrobial activity of indigenous wildly growing plants: potential source of green antibiotics. Afr. J. Microbiol. Res., 7(29): 3807-3815.
Meurer-Grimesa, B., McBetha, D.L., Hallihana, B., Delphab, S., 1996. Antimicrobial activity in medicinal plants of the Scrophulariaceae and Acanthaceae. Int.l J. Pharmacogn., 34(4): 243-248.
Newman, D.J., Cragg, G.M., Snader, K.M., 2000. The influence of natural products upon drug discovery. Nat. Prod. Rep., 17(3): 215-234.
Perez, C., Pauli M., Bazevque, P., 1990. Anantibiotic assay by the agar well diffusionmethod. Acta Biol. Med. Exp., 15: 113-115.
Shakya, M.N., Pradhan, R., Ranjitkar, R., 2008. A preliminary screening of some Nepalese medicinal plants for antimicrobial activity. Bulletin Depart. Plant Res., 30: 87-94.
Suffredini, I.B., Sader, H.S., Gonçalves, A.G., Reis, A.O., Gales, A.C., Varella, A.D., Younes, R.N., 2004. Screening of antibacterial extracts from plants native to the Brazilian Amazon Rain Forest and Atlantic Forest. Braz. J. Med. Biol. Res., 37(3): 379-384.