Association of Serum Neopterin Level with HCV Infection among Egyptian Blood Donors
1Microbiology Department, Faculty of Medicine, Minia University.
2Tropical Medicine, Faculty of Medicine, Cairo University.
3Biochemistry Department, Faculty of Pharmacy (Girls), Al-Alzhar University.
4Faculty of Pharmacy, Al-Alzhar University.
*Corresponding author:
Shahenda Nasr Ahmed;Email:
shahendan_ahmed@yahoo.comViews 3042
Abstract
The determination of neopterin is a new method for monitoring diseases which are linked with the activation of the immunity. This study was done to verify serum neopterin concentrations as a marker of virological infection with hepatitis C virus (genotype 4) in Egyptian blood donors. A total of 88 blood samples were collected from VACSERA blood bank by venipuncture and serum was obtained by centrifugation. Serum antibodies against Human Immunodeficiency Virus (HIV-1&2), Hepatitis C Virus (HCV Ab and PCR), and Hepatitis B Virus surface antigen (HBsAg) were determined in all samples by routine ELISA method. Serum neopterin was measured by a commercially neopterin enzyme Immunoassay kit. The diagnostic accuracy of marker was assessed using receiver-operating characteristic (ROC) curve analysis, describing the area under the curve (AUC) and its 95% confidence interval (CI). The samples were classified into two groups: Group I: 44 blood sample with HCV negative. Group II: 44 normal blood sample positive to HCV. Results showed that 9.09% HCV positive donors while 15.90% control group donors had increased neopterin level. Mean serum neopterin levels were elevated in healthy group (6.419 nmol/l) comparable to the HCV positive group (5.78 nmol/l) (P ˃ 0.05). When HCV patients were compared to healthy group, AUC for neopterin and HCV Ab were 0.526. Based on the ROC analysis, there were no adequate cut-off values for neopterin that would be best for differentiation of HCV from the non HCV patients. It is concluded that Neopterin assay isn’t a good diagnostic marker for HCV in blood donors.
Keywords
Neopterin; hepatitis C; blood donors; marker.
Citation
Abdel-Hamid, M., Ibrahim, Y.S., Ellakwa, D.E., Ahmed, S.N., 2016. Association of Serum Neopterin Level with HCV Infection among Egyptian Blood Donors. PSM Biol. Res., 01(1): 39-42.
Introduction
Hepatitis C is a blood-borne virus where it attributed as non-A/non-B hepatitis. The virus attacks cells in the liver, multiplies (replicates) (Andradeet al., 2009). There are estimates of 3% of the world’s population being chronically infected with HCV, that is the main source of liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) (Mohamed andAttalah., 2014). The risk factors linked with HCC comprise age, sex, diet, alcohol, and hepatitis B virus (HBV) and/or hepatitis C virus (HCV) infection (Ali et al., 2015).
Only in case of known diseases, there are specific tests available for detection of microorganisms. There was a remarkable progress in regard to safety of blood transfusion either by serologic or molecular biology techniques aiding blood donor screening, screening of the blood donors for elevated levels of liver enzymes in the blood for hepatitis, followed by Hepatitis B and then Hepatitis C screening was done (Shameem Banu et al., 2011).
Neopterin screening of blood transfusions became mandatory in Austria. Increased neopterin concentrations are helpful for the detection of subclinical infections or silent systemic disorders (Eisenhut., 2013).
Neopterin serum levels above 10 nmol/L are considered as elevated. Therefore, the assessment of neopterin in blood samples is a convenient tool in order to diminish the risk of infections via blood transfusion. Thereby it has to be noted that neopterin levels are already raised in an early stage of an infection, even before specific antibodies are produced to be able to further close the so-called “window period” which still prevail when antibody screening is done (Inci Fisenk et al.,2005;Shameem Banu et al., 2011).
The purpose of this study was to demonstrate association of serum Neopterin level with HCV infection among Egyptian blood donors.
Materials and Methods
This study was organized using 88 blood samples collected from blood bank of VACSERA in 2013,all samples were screened for HCV antibodies and PCR, hepatitis B surface antigen, hepatitis B core antibodies, HIV-1 and-2 antibodies. The two groups all of matched sex.
Accordingly, the samples were divided into 2 groups: Group I: include 44 donors (male aged between 19-45 years and negative for HIV antibody, hepatitis B surface antigen, hepatitis B core antibodies, HCV by antibodies and PCR). Group II: include 44 donors (male aged between 21-45 years and negative for HIV antibody, hepatitis B surface antigen, hepatitis B core antibodies, but positive for HCV by HCV antibodies and PCR). Biochemical tests for ALT and ALP determination were performed following procedures used by Muhammad et al. (2013).
A solid phase enzyme linked immunosorbent assay (ELISA), (according to Wachter et al. 1992, kits provided by IBL International GMBH)based on key assumption of a competitive ELISA was used for the in-vitro-diagnostic quantitative evaluation of neopterin level in human serum. The data obtained was evaluated statistically using SPSS version 16 and differences were assumed significant if the P-value was less than 0.05 (P < 0.05). An independent t-test, paired t-test and Spearman’s rho correlation was used to correlate different variables.
Results and Discussion
Our results showed that mean serum value for both AST and ALT in HCV positive group was non-significantly higher than those of the control group with mean serum value of (30.54 ± 9.9IU/L) and (27.54 ±9.9 IU/L) respectively at P > 0.05. The results showed significantly higher (P < 0.05) age of HCV positive group than the control group (Table 1). Rukiye and Fikriye (2016) have demonstrated higher levels of AST and ALT in HCV patients than healthy patients that support our findings. In a previous study mean values of serum ALT levels (82.7 ± 20.6 IU/L) and AST (79.2 ± 21.7 IU/L) were determined in HCV persistent group, while in control group mean values of serum ALT were 33.6 ± 10.7 IU/L and AST 32.6 ± 11.4 IU/L (Tabll et al., 2011). Significantly higher (P < 0.001) serum concentrations of AST and ALT were found in HCV-monoinfected subjects than in those who experienced spontaneous recovery from HCV. Similarly, higher serum AST ((P < 0.001)) and ALT ((P = 0.060)) concentrations were found in HIV-positive compared to HIV-negative HCV patients (Huang et al., 2016).
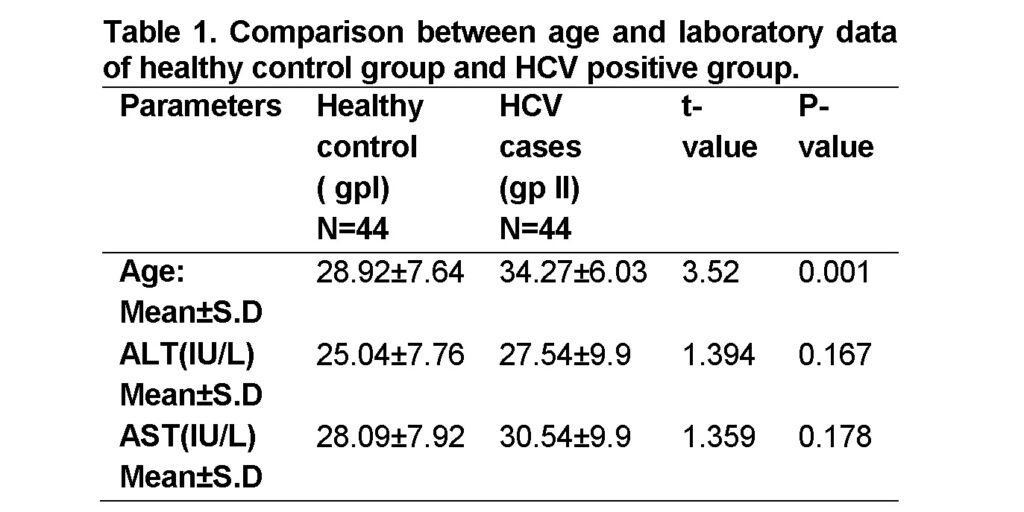
In this study 9.09% donors (4 of 44) from HCV positive group while 15.90% donors (7 of 44) from control group showed increased neopterin level. The mean neopterin concentration was higher in healthy control group (6.419±5.012 nmol/L) than in HCV positive group (5.78± 3.75 nmol/L) with statistically non-significant difference between the groups (p -Value > 0.05) (Table 2). Higher serum neopterin levels have been found to be linked with various diseases including rejection and viral infections (Chin et al., 2008; Grebe et al., 2011). Our findings are against Zoller et al. (2015) who determined neopterin concentrations to be 7.8 ± 8.7 nmol/L and greater than normal (p < 0.05) in HCV patients.
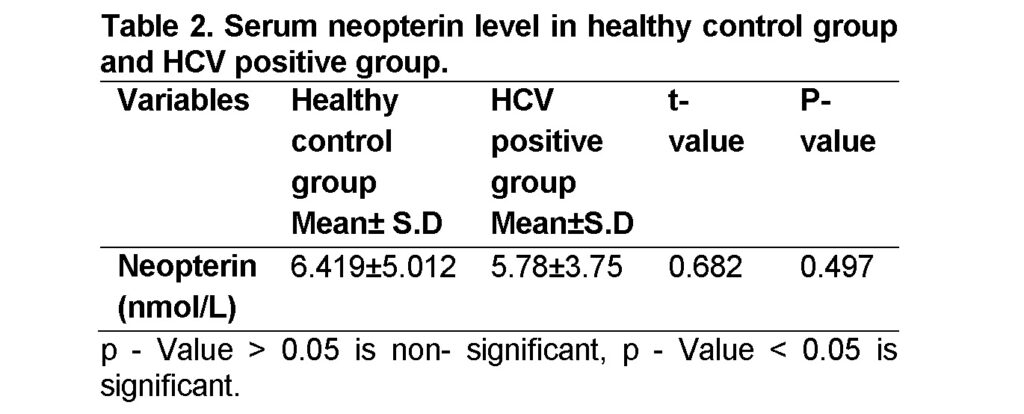
The correlation between age, ALT and AST in the healthy control group and the HCV positive group according to neopterin level showed non-significant difference between the groups, P > 0.05 (Table 3). From our study we did not find correlation between high neopterin concentrations and HCV antibody seropositive donations and no significant correlation between neopterin level and any studied variables (ALT, AST and age). Tabll et al. (2011) have demonstrated serum ALT and AST levels were significantly higher in group HCV positive patients than in control group (P value < 0.001).
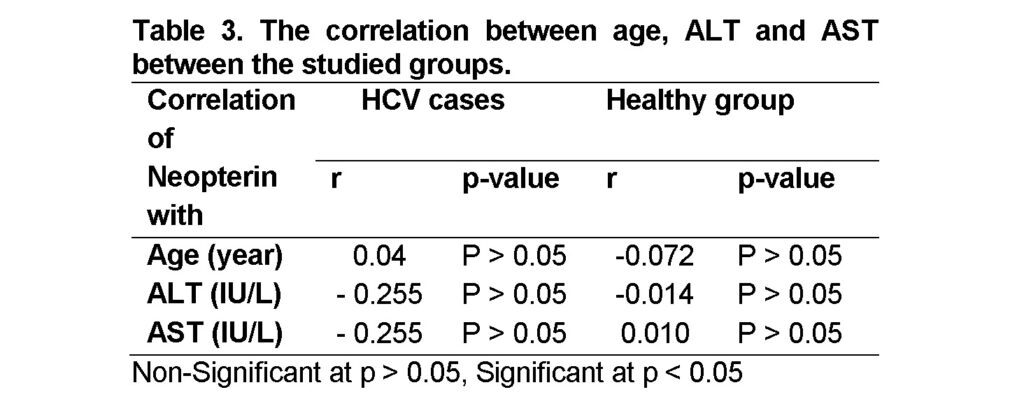
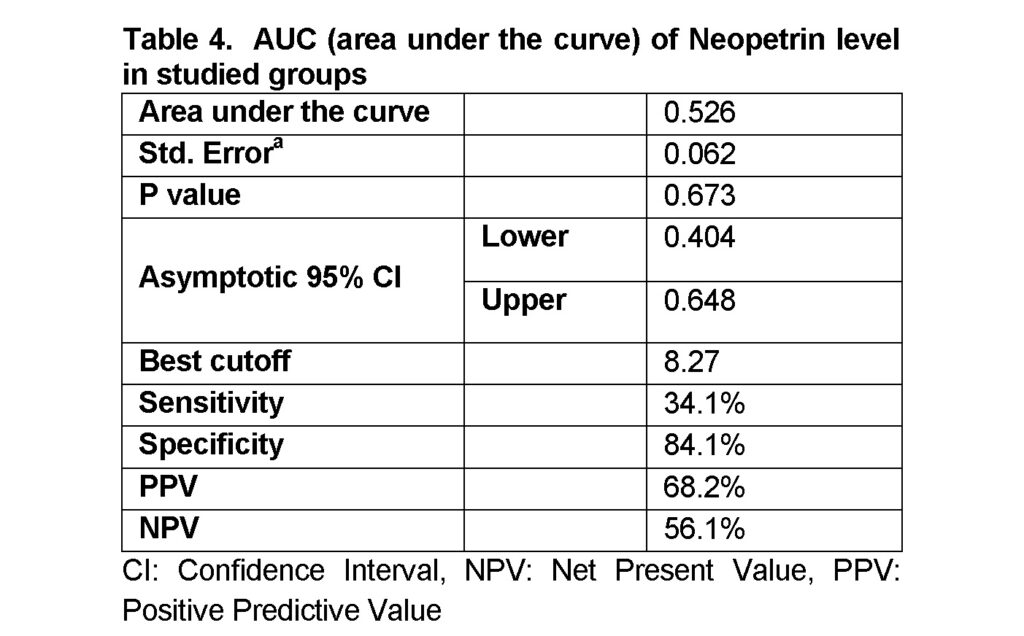
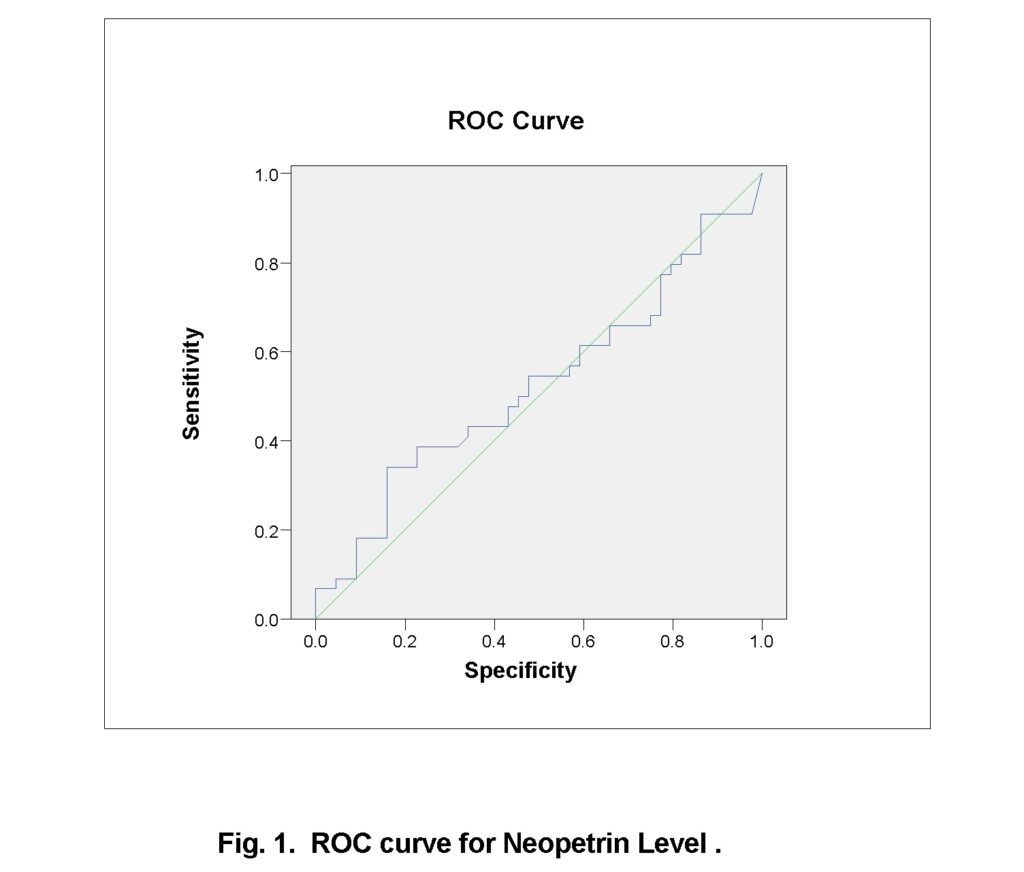
Table 4 and figure 1 showed that the receiver operating characteristic curve (ROC curve) showed that there is no significant area under the curve and neopterin is not a good marker to predict the infection of hepatitis C with specificity of 84.1%, sensitivity of 34.1%, PPV of 68.2% and NPV of 56.1% for diagnosis of HCV. In a previous study it was illustrated that a cutoff value of serum neopterin > 18.68 nmol/L (AUC=0.695) anticipated sensitivity 78.3%, specificity 58.4%, PPV 69.6% and NPV 82.6% (P=0.001) in HCV patients (Altonbary et al., 2015).
In the present study the seven samples which had elevated neopterin values but were not positive for any one of the screening tests, raised the issue of keeping the safety of the patients, so these seven samples should not be used for transfusion. So the present study further supports that increased neopterin concentrations in seronegative blood donations may be a marker for other unknown infections not screened for and transmission of these infections.
Conclusion and Suggestions
In conclusion neopterin is a nonspecific marker for screening of HCV infection in blood donors may be a useful marker for monitoring of infectious disease activity during treatment.
Also the neopterin assay thus detects a variety of potentially harmful diseases or conditions which would not be revealed by the usually employed battery of routine tests.
Hence we conclude that the risk of transmitting new pathogens may be reduced using neopterin assay as a routine work in our blood banks.
Acknowledgement
The authors are thankful to Prof. Dr. Mohamed Abdel-Hamid, Professor and Chairman, Department of Microbiology, Faculty of Medicine, El Minia University, Ass. Prof. Dr.Yasmin Saad Ibrahim, Faculty of Medicine, Cairo University, Dr. Doha EL-Sayed, Faculty of Pharmacy (Girls), Al-Azhar University for great help throughout the work, fruitful guidance, brilliant and creative remarks that helped this modest work to be produced in this form and helped me in the final accomplishment during the supervision of the work.
Conflict of Interest
There is no conflict of interest.
References
Altonbary, A., Attwa, M., Bahgat, M., Abdel-Khalek., Elghannam, D., Saudy, N., 2015. Complementary Role of Neopterin and the IL-28B Polymorphism in Predicting Antiviral Response in Genotype 4 Chronic Hepatitis C Virus Patients. Glob. J. Gastroenterol. Hepatol., 3: 59-65.
Ali, H.M., Bhatti, S., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., Muhammad, A., 2015. Mutational analysis of MDM2 gene in hepatocellular carcinoma. Sci. Lett., 3(1):33-36.
Andrade, L.J.D., D’Oliveira, A., Melo, R.C., De Souza, E.C., Silva, C.A.C., Parana, R., 2009. Association between hepatitis C and hepatocellular carcinoma. J. Global Infect. Dis., 1(1): 33-37.
Chin, G.K., Adams, C.L., Carey, B.S., Shaw, S., Tse, W.Y., Kaminski, E.R., 2008. The value of serum neopterin, interferon-gamma levels and interleukin-12B polymorphisms in predicting acute renal allograft rejection. Clin. Exp. Immunol., 152: 239-44.
Eisenhut, M., 2013. Neopterin in Diagnosis and Monitoring of Infectious Diseases. Journal of Biomarkers; 2013 (2013): Article ID 196432, 10 pages.
Grebe, S.O., Kuhlmann, U., Fogl, D., Luyckx, V.A., Mueller, T.F., 2011. Macrophage activation is associated with poorer long-term outcomes in renal transplant patients. Clin. Transplant., 25(5): 744-754. doi: 10.1111/j.1399-0012.2010.01345.x
Huang, X., Liang, H., Fan, X., Zhu, L., Shen, T., 2016. Liver Damage in Patients with HCV/HIV Coinfection Is Linked to HIV-Related Oxidative Stress. Oxid. Med. Cell. Longev., Article ID 8142431, 11 pages. http://dx.doi.org/10.1155/2016/8142431
Inci Fisenk, B., US, D., Ozcebe, O.I., Hascelik, G., 2005. The value of increased Neopterin levels in reducing transfusion-transmitted virus infections: Detection of a donation from a HbsAg positive chronic carrier by screening of neopterin in Turkish blood donors. Scand. .J Infec. Dis., 37:599-604.
Mohamed, S.M., Attalah, M.F., 2014. Prediction of response to pegylated interferon and ribavirin in Egyptian patients with chronic hepatitis C using serum neopterin. Egyptian Liver J., 4(2): 63-68.
Muhammad, A., Farooq, M.U., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., 2013. Prevalence of diabetes mellitus type II in patients with hepatitis C and association with other risk factors. Punjab Univ. J. Zool., 28 (2): 69-75.
Rukiye, N.A.R., Fikriye, M.S., 2016. The Relationship Between the Serum RNA Titers of Hepatitis C Virus and Biochemical Parameters in Chronic Hepatitis C Patients. Viral Hepat. J., 22: 28-33.
Shameem Banu, A.S., Latha, P., Kaveri, K., Jayakumar, S., 2011. Serum Neopterin Estimation as an Indicator for Safe Blood Transfusion. J. Clin. Diagn. Res., 5(8): 1555-1558.
Wachter, H., Fuchs, D., Hausen, A., Reibnegger, G., Weiss, G., Werner, E.R., Werner-Felmayer, G., 1992. Neopterin:Biochemistry – Methods – Clinical Application. Cell Biochemistry and Function; 10(4): 289.
Zoller, H., Jenal, A., Staettermayer, A.F., Schroecksnadel, S., Ferenci, P., Fuchs, D., 2015. Tryptophan Breakdown in Patients with HCV Infection is Influenced by IL28B Polymorphism. Pharm., 8(2): 337-350.