A Comparative Study on Isolation and Identification of Bacillus thuringiensis from Different Localities of Gujranwala City
1Department of Zoology, Lahore College for Women University, Jail Road Lahore 54000, Pakistan.
2The School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China.
3Pakistan Science Mission (PSM), Noor Kot 51770, Pakistan.
*Corresponding author:
Muhammad Naeem Iqbal;Email:
driqbalnaeem@hotmail.comViews 3412
Abstract
This was a comparative study on isolation and identification of Bacillus thuringiensis isolates from different localities of city Gujranwala. Isolates of B. thuringiensis were identified using microscopic characters, colony morphology and biochemical characters. Then SDS-PAGE was performed to obtain a high protein profile on the basis of Cry proteins. A total of 74 out of 100 samples were found positive with B. thuringiensis. The maximum diversity of isolates was present in the soil (25%) as compared to other samples like dust (23.8%), cow dung (17.5%), donkey dung (17.5%) and bird droppings (16.2%). The average value of bacterial load was highest in soil (1 ± 0.192 CFU/ml) and lowest in bird droppings (0.650 ± 0.131 CFU/ml). The different sizes of bands with representative nine isolates from all sample sources on SDS-PAGE represent characteristics of crystal proteins having different target order specificity. It was concluded that B. thuringiensis is prevalent in various localities of the city. The need of the hour is to characterize these isolates and use them for pesticidal activity.
Keywords
Bacillus thuringiensis, Cry proteins, SDS-PAGE, biopesticide.
Citation
Yunus, F.N., Kanwal, F., Rashid, F., Ashraf, A., Iqbal, M.N., Xiao, S., 2016. A Comparative Study on Isolation and Identification of Bacillus thuringiensis from Different Localities of Gujranwala City. PSM Biol. Res., 01(1): 34-38.
Introduction
Agriculture and forests are important resources which prolong the social, economical and ecological systems. The main concern is to protect these resources against the pests, but due to undesirable effects of chemical insecticides, biopesticides have been used greatly (Marrone, 1999). Most commonly, all animal species are being regulated by other living organisms known as antagonists that are present naturally and this phenomenon is known as Natural Biological Control (NBC).
Bacillus thuringiensis is a type of bacteria which is Gram positive and produces spores during sporulation. It has rod shape and it is about 1µm in width and 5 µm in length (Sakai et al., 2007). The genome size of B. thuringiensis ranges from a minimum of 2.4 to a maximum of 5.7 million bp. Many isolates contain numerous extra chromosomal parts which contain both globular as well as linear shapes (Carlson et al., 1994). There are almost 34 subspecies (also called serotypes or varieties) of B. thuringiensis and possibly over 800 strains or isolates (Lambert and Peferoen, 1992). Biopesticides based on the B. thuringiensis are extremely important and their percentage in the world’s biopesticide market is about ninety seven percent (Cannon, 1993).
Depending upon the composition of protoxin, crystals have numerous shapes such as Cry1 having bipyramidal, Cry2 having cuboidal, Cry3A having flat rectangular, Cry3B having irregular, Cry4A and Cry4B having spherical and Cry11A having rhomboidal shapes (Schnepf et al., 1998). Any particular bioactivity by B. thuringiensis is controlled via insecticidal crystalline proteins (ICPs) which are being codified in the specific genes known as cry genes so they are vulnerable towards specific insects like Coleopterans, Dipterans and Lepidopterans. Some other insect orders such as Homoptera, Hymenoptera, Dictyoptera and Mallophaga are also controlled by B. thuringiensis. In addition, several crystal toxins are also active against non-insect species such as roundworms, Acarid mites, Platyhelminthes along with protozoans (Marroquin et al., 2000). Both spores and ICPs produced by B. thuringiensis have been used to control insect pests since the 1920s (Peggy, 2008). Each proteolytically activated ICP molecule having insecticidal activity has two terminal domains. One of the domains is C-terminal that is unpredictable and specific for the identification of receptor of their hosts and other is an N- terminal domain that contains preserved sequences so brings toxicity by causing minute openings in the gut of insects (Li et al., 1991).
Since the first cry gene was cloned from B. thuringiensis then sequenced and expressed in Escherichia coli in 1981, more than 335 different genes have been described (Lecadet et al., 1999). From an ecological point of view natural habitat of B. thuringiensis is not exactly known. Historically, it has been found in soil, sick or deceased pest and stored food like tobacco, grain and flour (Hongyu et al., 2000) although it is discovered in dust, in deferral, on plant leaves, in food, animal skin and in the aquatic environment (Meadows et al., 1992; Akhurst et al., 1997; Bernhard et al., 1997; Smith and Barry, 1998; Maeda et al., 2000).
The present work was conducted to isolate and identify B. thuringiensis from different localities of city Gujranwala in order to describe the vast diversity of B. thuringiensis.
Materials and Methods
Sample processing technique for the isolation of B. thuringiensis
There were hundred samples collected from twenty different localities, including twenty numbers of samples for each of the five types of sample sources (soil, stored product dust, cow dung, donkey dung and bird droppings). LB (Lauria birtani) media was used for sample processing. The technique of Martin and Travers (1989) as described by Makhdoom, (1997) was used with certain modifications for isolating B. thuringiensis. One gram of each sample was weighed and resuspended in 10ml LB broth medium in test tubes, then vortexed for five minutes until a homogenized mixture was obtained. Kept the test tubes for 30 min. at room temperature, then filtered them in order to collect the supernatant. Tenfold serial dilutions (10-1 to10-6)were prepared from the supernatants in eppendorfs adding up of 100µl supernatant and 900µl of distilled water. Then placed the dilutions in a hot water bath to give heat shock at 80ºC for 10 min. so that to isolate B. thuringiensis. Then poured up to 200µl of samples on LB agar plates and spread with spreader, incubated them at 32 ºC for 24 hours.
Screening of B. thuringiensis
The growth of B. thuringiensis best occurs in LB medium, so LB medium was used in order to study the colony morphology of the isolates. After 24 hours, the growth on LB-agar plates was observed and those colonies were selected which contained morphology apparently like that of B. thuringiensis. After the growth observation of bacterial colonies, the colonies were counted under a colony counter as colony forming units per ml (CFU/ml) of sample (Iqbal et al., 2015). The colonies were appeared with different diameters ranging from 2mm to 8-10mm in diameter and the colonies with B. thuringiensis like morphologies were streaked again on the sterile LB-agar plates, then incubated at 32ºC for 24 hr. After incubation, LB-plates appeared with different characteristic morphologies relative to the origin of their isolates and thus pure cultures were obtained in the form of single colonies. Bacillus isolates were subjected to Gram (Gram, 1884), endospore (Crystal protein staining) and biochemical tests like starch hydrolysis (Karnataka, 2009: Iqbal et al., 2015) following Bergey’s Manual of Determinative Bacteriology (Bergey, 1984).
Toxin extraction from B. thuringiensis
For toxic crystal protein extraction from B. Thuringiensis isolates, LB agar plates were streaked with fresh 24 hour stab culture in a crisscross manner, then kept the petri plates in the incubator for 72 hours at 32-37ºC. After incubation, heavy growth of pure cultures of B. thuringiensis isolates was obtained. The petri plates were flooded with 5-10 ml of cold distilled water for gently scraping off the growth of Bacillus with the help of a sterile toothpick then poured the water of petri plates having B. thuringiensis isolates in sterile eppendorfs. Kept the eppendorfs on ice and centrifuged at 4000 rpm for 10 min. then collected the supernatant. Again kept the eppendorfs on the ice after that heat shock was done by keeping the collected supernatant on a boiling water bath for 6 min. then kept it on the ice thus, crystal protein was extracted from B. thuringiensis isolates.
SDS-PAGE
Bio-Rad Mini protein 11 gel apparatus was used to perform SDS-PAGE (Laemmli, 1970). The crystal protein was mixed with the bromophenol dye by taking 20µl of purified crude toxin protein, mixed it with 5µl of dye for loading in wells of 10% SDS-PAGE (4 % stacking gel and 10 % resolving gel). The protein marker was loaded in first well and rests of nine wells were loaded by toxin proteins of B. thuringiensis isolates from five types of sample sources. Then an electric field was applied and movement of proteins was observed, followed by formation of bands of different molecular weights. Allowed the gel to run for one and half an hour after adding Coomassie Brilliant blue stain, then kept it on the shaker for thirty minutes and destained the gel with destaining solutions until the blue back ground disappeared. Then observed the bands under UV light and photographed.
Statistical analysis
For the statistical analysis of results, including Standard error mean of all the B. thuringiensis isolates, Minitab version 14 was used. Analysis of variance (ANOVA) was carried out by means of SPSS version 16 to investigate and model the relationship among variables and the results were shown in the form of graphs and tables.
Results
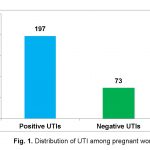
The results showed that seventy four sampleswere positive for B. thuringiensis out of hundred samples. Among these, thirty three samples were present with heavy B. thuringiensis on the basis of best crystal protein production. The frequency of B. thuringiensis isolates also varied in different localities relative to the diversity of isolates in those localities.
For determining the morphological characterization, all the isolates of B. thuringiensis isolated from various selected samples were cultured on the LB-agar medium. After 24 hr samples were observed and a total of eighty isolates were identified through microscopy. The isolates which appeared with different characteristics as described for B. thuringiensis isolates were rejected based on the difference in morphological characteristics from B. thuringiensis. The maximum diversity of isolates was present in the soil (25%) as compared to other samples like dust (23.8%), cow dung (17.5%), donkey dung (17.5%) and bird droppings (16.2%) (Table 1) and frequency of B. thuringiensis also varies according to their diversity in those samples. The average microbial load of all samples collected from different localities was determined. The average value of soil was 1 ± 0.192 CFU/ml, dust 0.950 ± 0.135 CFU/ml, cow dung 0.700 ± 0.147 CFU/ml, donkey dung 0.700 ± 0.164 CFU/ml, bird droppings 0.650 ± 0.131 CFU/ml (Table 1).
Protein profile of B. thuringiensis isolates
Nine representative B. thuringiensis isolates of various sample sources were further screened for the presence of the best crystal proteins and they were analyzed through SDS-PAGE to obtain a gel pattern of isolates which was compared with high molecular weight marker band. Coomassie brilliant blue (staining dye) visualized cleared bands of different molecular weights of all isolates. The different sizes of bands represent characteristics of crystal proteins having different target order specificity (Figure 1). It was observed that protein profiles of isolates collected from the same sample source were very similar than those from different sample sources. Results obtained by ANOVA showed significant (P<0.05) variation (F 4, 95 = 3.827), P=0.006 and Tukey’s post hoc test gave two homogeneous subsets.
Discussion
Hundred different samples including soil, stored product dust, cow dung, donkey dung and bird droppings were practiced in order to identify the presence of B. thuringiensis and 74 samples were positive. Similarly previous studies have also reported isolation of B. thuringiensis from soil (Shishir et al., 2012; El-Didamony, 2014). For screening of B. thuringiensis different techniques were used and the selection of Bacillus isolates was on the bases of their similarities to that of B. thuringiensis. A combine action of cooperative and competitive behavior enhances the bacteria for establishing the colonies of different shapes that show the development of isolation in a population which are similar to each other and they are also present in semi-solid agar media (Shapiro, 1995; Wakano et al., 2003).
The exact ecology of B. thuringiensis is not known, this bacterium has worldwide distribution including soil, stored grains and the plant surfaces, but mostly it is considered as an opportunistic pathogen (Schnepf et al., 1998). Isolates of B. thuringiensis are collected worldwide and is also present in insect bodies as well as in their surroundings, dust of stores, residues of plants and in water (Bel et al., 1997).
In order to make the protein profile of B. thuringiensis isolates nine isolates were selected for SDS-PAGE analysis on the basis of best crystal protein production. The different sizes of bands represent characteristics of crystal proteins having different target order specificity. Buentello-Wong et al. (2015) characterized Cry proteins in native strains of Bacillus thuringiensis. A large number of B. thuringiensis isolates were reported from different ecological regions of Pakistan and they were characterized for the composition of crystal protein gene and their pesticidal activity against two rice insect pests of Lepidoptera, Scirpophaga incertulas (the yellow stem borer) and Cnaphalocrocis medinalis (the rice leaf folders). On the basis of initial screening, representing seventeen isolates were preferred and further characterized for pesticidal activity based on colony and parasporal inclusion morphology, SDS-PAGE, western blot analysis and comparative biotoxicity assays for determining the LC50. Parasporal inclusion bodies and spores were produced by all the isolates. Immunoblotting results showed that Pakistanian isolates synthesized entomocidal proteins that belong to toxin groups of Cry1A and Cry2A crystal proteins (Karim and Riazuddin, 1999).
Isolates of B. thuringiensis were also resolved in the region of Spain by means of SDS-PAGE accompanied by biological standardization of endotoxins action and effect towards various insects for the composition as well as ecological distribution of B. thuringiensis serotypes and crystal proteins (Moraga et al., 2004).
Acknowledgement
The authors are highly thankful to Dr. Fakhar-un-Nisa Yunus, Department of Zoology, Lahore College for Women University, Lahore, for technical support during this research work. The authors acknowledge that this work is from student thesis submitted in Higher Education Commission (HEC), Pakistan.
Conflict of interest
The authors confirm that this article content has no conflict of interest.
References
Akhurst, R.J., Lyess, E.W., Zhang, Q.Y., Cooper, D.J., Pinnock, D.E., 1997. A 16S r RNA gene oligonucleotide probe for identification of Bacillus thuringiensis isolates from sheep fleece. J. Invert. Pathol., 69: 24-31.
Bel, Y., Granero, F., Alberola, T.M., Sebastian, M.J., Ferre, J., 1997. Distribution, frequency and diversity of Bacillus thuringiensis in olive tree environments in Spain System.J. Appl. Microbiol., 20: 652-658.
Bergey, S.A., 1984. Bergey, Manual of Determinative Bacteriology, 9th edition, Williams & Wilkins., Philadelphia.
Bernhard, K., Jarret, P., Meadows, M., Butt, J., Ellis, D.J., Roberts, G.M., Pauli, S., Rodgers, P., Burges, H.D., 1997. Natural isolates of Bacillus thuringiensis: worldwide distribution, characterization and activity against insect pests. J. Invert. Pathol., 70(1): 59-68.
Buentello-Wong, S., Galan-Wong, L., Arevalo-Nino, K., Almaguer-Cantu, V., Rojas-Verde, G., 2015. Characterization of Cry Proteins in Native Strains of Bacillus thuringiensis and Activity against Anastrepha ludens. Southwest. Entomol., 40(1):15-24.
Cannon, R.J.C., 1993. Prospects and progress for Bacillus thuringiensis based pesticides. Pest. Sci., 37(4): 331-335.
Carlson, C.R., Caugant, D.A., Kolsto, A.B., 1994. Genotypic diversity among Bacillus cereus and Bacillus thuringiensis strains. Appl. Environ. Microbiol., 60(6): 1719-1725.
El-Didamony, G., 2014. Occurrence of Bacillus thuringiensis and their phages in Yemen soil. Virus dis., 25(1): 107-113.
GRAM, H. C 1884. Über die isolierte Färbung der Schizomyceten in Schnitt- und Trockenpräparaten (in German). Fortschr. Med. 2: 185-189.
Hongyu, Z., Ziniu, Y., Wangxi, D., 2000. Composition and ecological distribution of Cry proteins and their genotypes of Bacillus thuringiensis isolates from warehouses in China. J. Invert. Pathol., 76: 191-197.
Iqbal, M.N., Anjum, A.A., Ali, M.A., Hussain, F., ALI, S., Muhammad, A., Irfan, M., Ahmad, A., Irfan, M. and Shabbir, A., 2015. Assessment of microbial load of un-pasteurized fruit juices and in vitro antibacterial potential of honey against bacterial isolates. Open Microbiol. J., 9: 26-32. DOI: 10.2174/1874285820150601E001.
Karim, S., Riazuddin, S., 1999. Rice insect pest of Pakistan and their control. A lesson from past for sustainable future integrated pest management. Pak. J. Biol. Sci., 2(2): 261-276.
Karnataka, J.,2009. Distribution of Bacillus thuringiensis Berliner strains in the soils of different habitats and their activity against white grubs. J. Agri. Sci.,22(3): 628-630.
Laemmli, U.K., 1970. Cleavage of structure proteins during the assembly of the head of bacteriophage T4. Nature., 227(5259): 680-683.
Lambert, B., Peferoen, M., 1992. Insecticidal promise of Bacillus thuringiensis. Biosci., 42(2): 112-122.
Lecadet, M.M., Frachon, E., Dumanoir, V.C., Ripouteau, H., Hamon, S., Laurent, P., THIERY, I., 1999. Updating the H-antigen classification of Bacillus thuringiensis. J. Appl. Microbiol., 86(4): 660-672.
Li, J., Carrol, J., Ellar, D.J., 1991. Crystal structure of insecticidal delta-endotoxin from Bacillus thuringiensis at 2.5 Å resolutions. Nature., 353(6347): 815-821.
Maeda, M., Mizuki, E., Nakamura, Y., Hatano, T., Ohba, M., 2000. Recovery of Bacillus thuringiensis from marine sediments of Japan. Curr. Microbiol., 40(6): 418-422.
Makhdoom, R., 1997. Cloning and sequencing of the delta endotoxin gene from locally isolated Bacillus thuringiensis toxic against spotted bollworm. Ph.D. thesis. University of the Punjab, Lahore. 20-25.
Marrone, P.G., 1999. Microbial pesticides and natural products as alternatives. Agri. Outlook., 28(3): 149-154.
Marroquin, L.D., Elyassnia, D., Griffitts, J.S., Feitelson, J.S., Aroian, R.V., 2000. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genet. 155(4): 1693-1699.
Martin, P.A., Traverse, R.S., 1989. World-wide abundance and distribution of Bacillus thuirngiensis isolates. Appl. Environ. Microbiol., 55(10): 2437-2442.
Meadows, M.P., Ellis, D.J., Butt, J., Jarrett, P., Bruges, H.D., 1992. Distribution, frequency and diversity of Bacillus thuringiensis in an animal feed mill. Appl. Environ. Microbiol., 58(4): 1344-1350.
Moraga, E.Q., Tovar, E.G., Garcia, P.V., Alvarez, C., 2004. Isolation, geographical diversity and insecticidal activity of Bacillus thuringiensis from soils in Spain. Res. Microbiol. 159(1): 59-71.
Peggy, L., 2008. Genetically Engineered Plants and Foods: A Scientist’s Analysis of the Issues (Part I). Annu. Rev. Plant Biol., 59: 771-812.
Sakai, H., Howlader, M.T.H., Ishida, Y., Nakaguchi, A., Oka, K., Ohbayashi, K., Yamagiwa, M., Hayakawa, T., 2007. Flexibility and strictness in functional replacement of domain III of Cry insecticidal proteins from Bacillus thuringiensis. J. Biosci. Bioeng., 103(4): 381-383.
Schnepf, E., Crickmore, N., Rie, J.V., Lereclus, D.J., Baum, J., Feitelson, J., Zeigler, D.R., Dean, D.H., 1998. Bacillus thuringiensis and its Pesticidal Crystal Proteins. Microbiol. Mol. Biol. Rev. 62(3): 775-806.
Shapiro, J.A., 1995. The significances of bacterial colony patterns. BioEssays., 17: 597-607.
Shishir, A., Akter, A., Hassan, M.H., Kibria, G., Ilias, M., Khan, N.S., Hoq, M.M., 2012. Characterization of locally isolated Bacillus thuringiensis for the development of Eco-friendly Biopesticides in Bangladesh. J. Biopest., 5 (Supplementary): 216-222.
Smith, R.A., Barry, J.W., 1998. Environmental persistence of Bacillus thuringiensis spores following aerial application.J. Invert. Pathol., 71: 263-267.
Wakano, J.Y., Maenosono, S., Komoto, A., Eiha, N., Ymaguchi, Y., 2003. Self-organized pattern formation of a bacteria colony modeled by a reaction diffusion system and nucleation theory. Phys. Rev. Lett., 90(25): 258102.