Prevalence of Liver disorders in Islamabad City
1Department of Zoology, 2Department of Biochemistry, PMAS Arid Agriculture University, Rawalpindi 46000, Pakistan.
3Pakistan Science Mission (PSM), Noor Kot 51770, Pakistan.
4Department of Zoology, Lahore College for Women University, Lahore 54000, Pakistan.
5Department of Anatomy and Histology, 6Department of Wildlife and Ecology, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan.
7Department of Zoology, University of Education, Township Lahore 54000, Pakistan.
8Department of Zoology, University of Poonch, Rawalakot 12350, Azad Kashmir, Pakistan.
*Corresponding author:
Asfa Ashraf;Email:
sundausnaeem@yahoo.comViews 3050
Abstract
The purpose of this study was to determine the prevalence of liver disorders through liver function enzyme tests (LFTs) in Islamabad. A total of 250 blood samples were screened in Kahuta Research Laboratory (KRL), Islamabad for the presence of liver disorders through liver function enzyme tests (LFTs) viz., alanine aminotransferase (ALT), Total Bilirubin (T.Bil) and alkaline phosphatase (ALP). The results were analyzed by comparing the correlation among ALT, ALP and T. Bilirubin and compiled by comparing elevated level of LF enzymes with respect to age. It has been observed that correlation coefficient between ALT and T. Bilirubin; ALP and T. Bilirubin; ALT and ALP was positive values which indicate that slightly elevation in these enzymes released from hepatocytes cause liver disorders. The level of ALT and ALP abruptly decreased in (1-15 years), and then it gradually increased in age groups 16-30 years, 31-45 years. Their level was much higher in (46-60 years). However the level of T. Bilirubin was higher in children and adults in age groups (1-15 years) and (46-60 years) but it was slightly elevated in age groups (16-30 years) and (31-45 years). It is concluded that elevation of liver enzymes is responsible for liver disorders.
Keywords
Liver function test, Liver disorders in Islamabad region of Pakistan.
Citation
Toor, S., Toor, S., Ashraf, A., Alam, S., Anwaar, S., Saddiqa, A., Ali, S., Muhammad, A., Toor, S., Akhter, S., 2016. Prevalence of Liver disorders in Islamabad City. PSM Biol. Res., 01(1): 31-33.
Introduction
Liver performs its vital functions such as synthesis, storage, recycling and detoxification under normal conditions. There are number of factors involved in the elevation of liver enzymes. Previous studies reported risk factors included parental drug abuse (Marengo, 1993), blood transfusion (Dusheiko, 1990), accidental needle stick (Kiyosawa et al., 1991), ear piercing, bite by human (Abildgaard and Petrsland, 1991), haematophagous arthropods such as mosquito, ticks and bedbugs (Hollinger, 1990), organ recipients (Pereia et al., 1991), obesity (fatty deposits in liver cells) (Fattovich et al., 1997). Urine, saliva and blood samples used to detect sick factor of liver disorder (Dubios et al., 1994). Liver disorders including Hepatitis A, B, C, D, E and G, liver tumors, liver stones, liver cancer and obstructions of bile duct due to stones (Stanley, 1996).
It has been observed that liver diseases also induced by antibiotics such as Nitrofuranation, Amoxicillin, Clavulanic acid, Tetracycline, Isoniazid and Methotrexate used to treat autoimmune disorders and cancer. Disulfiram used to treat alcoholics can also cause liver inflammation (Seng and Katzel, 2001). Numerous medications cause liver inflammation including taking excess amount of Acetaminophen (Schalm et al., 1997). Niacin is used to control elevated blood level of cholesterol, but liver inflammation for this medication is related to the dose taken (Prince et al., 2002).
Liver function enzyme tests (LFTs) including Alanine transaminase (ALT), Aspartate transaminase (AST), Alkaline phosphatase (ALP), Albumin, Total bilirubin (T.BIL), Direct bilirubin and Gamma glutamyl transpeptidase (GGT) tests are used as a marker to determine the status of patient’s liver (Haurigan and Bowling, 2001). Medical treatments for liver disorder include surgery, low sodium diet and water pills (diuretics), anti-inflammatory drugs and liver transplantation (Schalm et al., 1997). The main objective of this study was to diagnose and determine the prevalence of liver diseases through liver function enzyme tests (LFTs) in Islamabad, Pakistan.
Materials and Methods
The study was conducted in patients visiting Outdoor Patients Department (OPD) of Kahuta Research Laboratory (KRL) Islamabad for period of five months from September 2012 to January 2013. A total of 250 patients were screened for the presence of liver disorders. A survey was conducted to access the prevalence of liver disorders through liver function enzyme tests (LFTs) viz., alanine aminotransferase (ALT), T. bilirubin, and alkaline phosphatase (ALP) and other information about sex, age, occupation, locality, personal history and socioeconomic status were collected on prescribed questionnaire. Blood samples were collected in 5 ml EDTA coating vacutainers with the help of hypodermic syringe. Blood samples were centrifuged at 3000 rpm for 15 minutes to collect the serum and then serum was stored at 4°C for biochemical analysis. Biochemical tests for ALT, ALP and Bilirubin determination were performed following procedures used by Muhammad et al. (2013). The data was arranged in various groups and was analyzed through Correlation using SPSS version 16.
Results and Discussion
During this study, a total of 250 blood samples consisting of 123 (49.2%) males and 127 (50.8%) females attending Kahuta Research Laboratory (KRL) Islamabad were screened for the presence of liver disorders. All the patients were found with general signs and symptoms related to liver abnormalities such as indigestion, fatigue, fever, weakness, loss of appetite, abdominal pain, dark urine and yellow eyes.
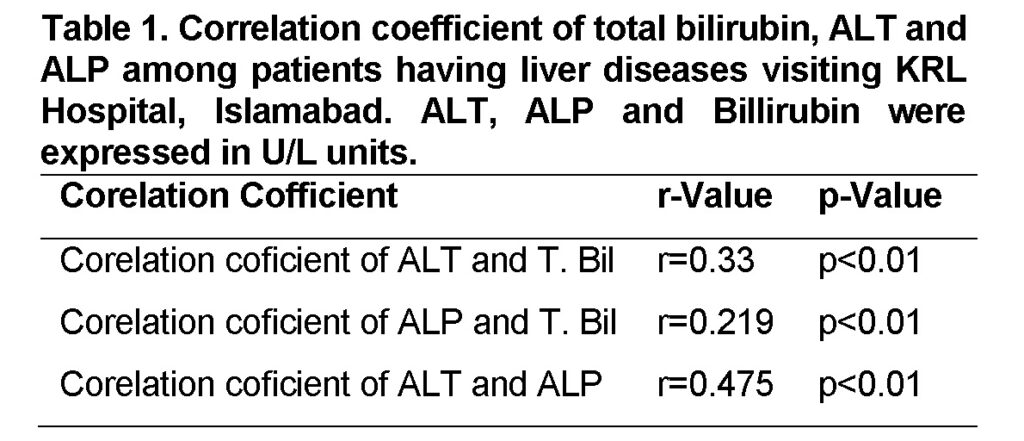
Corelation coficient of ALT and T. Bilirubin
The Alanine amino transaminase (ALT) showed a statistically significant positive correlation (r=0.33, p<0.01) with T. bilirubin shown in (Table 1). These results are similar to Loannou et al. (2006), who showed that the level of Alanine amino transaminase (ALT) and T. Bilirubin in hepatic tissues abruptly rise especially when liver tissues are damaged due to HCV.
Corelation coficient of ALP and T. Bilirubin
The Alkaline phosphatase (ALP) also showed a statistically positive correlation (r=0.219; p<0.01) with T. bilirubin (Table 1). Level of Alkaline phosphatase (ALP) in relation with T. Bilirubin becomes higher during bile duct obstruction and intra-hepatic cholestasis (Schafer and Sorrell, 1997).
Corelation coficient of ALT and ALP
In present study, Alanine transaminase (ALT) showed a statistically significant positive correlation with Alkaline phosphatase (ALP) (r=0.475; P<0.01) shown in (Table 1). Similar results were reported by previous studies. The level of ALT with respect to ALP has become a well-known marker in inflammatory necrosis in patients with chronic liver failure (Tarao et al., 2002). ALT level in relation with ALP level estimation considered as the most important test for recognition of acute and chronic hepatic injuries and thus meet clinical monitoring patient with chronic hepatic injuries (Dufour et al., 2001).
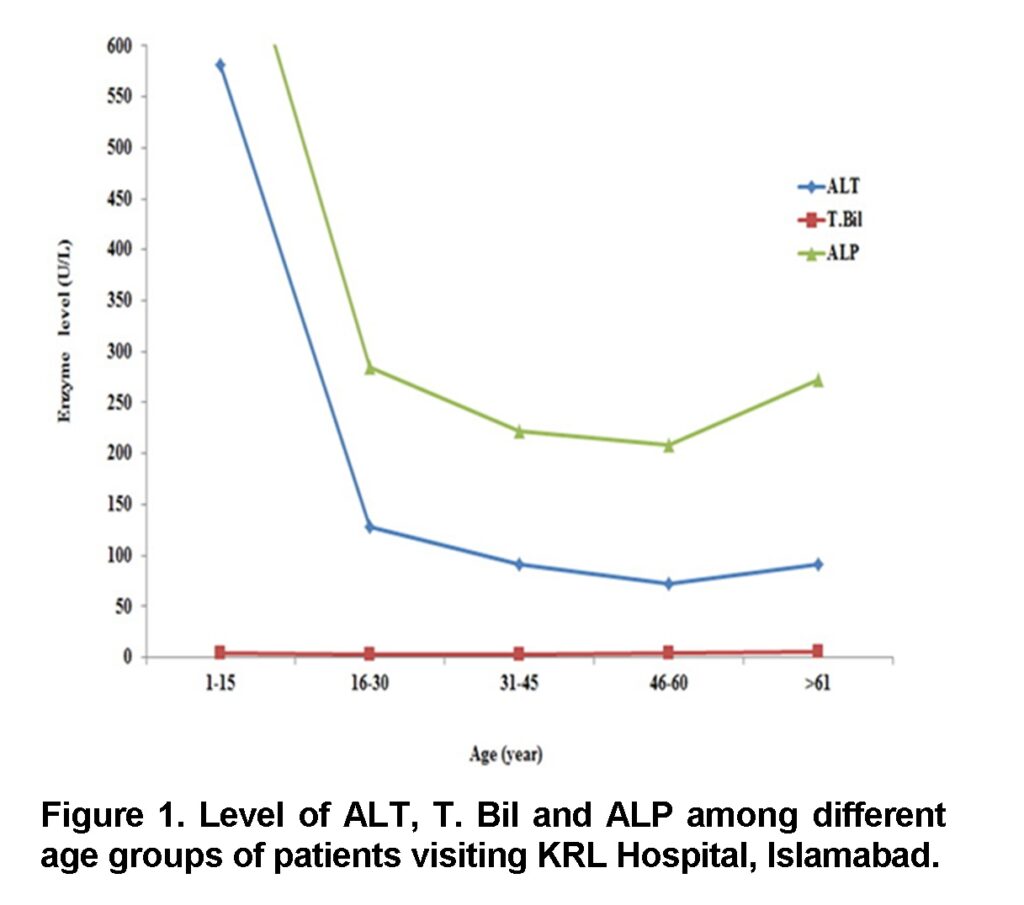
Elevated level of LF-Enzymes with respect to age
It has been observed that the level of ALT and ALP abruptly decreases in (1-15 years), and then it gradually increases in age groups (16-30 years), (31-45 years). Their level is much higher in (46-60 years). However the level of T. Bilirubin is higher in Children and adults in age groups (1-15 years) and (46-60 years) but its level is slightly elevated in age groups (16-30 years) and (31-45 years) (Figure 1).
Previous studies have also shown similar results. Findings of Amarapukar (2000) showed that progression to cirrhosis is faster in those who acquired infections after the age of 35 years. Muhammad et al. (2013) found that HCV was more prevalent in women (60.4%) than in men (39.6%), with the highest incidence (28%) in age group 24-34 years. Similar results are also highlighted by Lucidarme et al., (1998) who had reported that the progression to Hepatocellular carcinoma (HCC) is slower in patients before 30 years of age as compare to older age. Ali et al. (2014) documented age, sex, diet, alcohol, and infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) as the risk factors linked with HCC. It was found that point mutation at MDM2-SNP309 gene from T to G may be responsible for HCC in patients with HCV.
Conclusion and Suggestions
It is concluded that elevation of liver enzymes is responsible for liver disorders. It is recommended to avoid those medications which cause liver inflammation responsible for excess release of liver enzymes. Epidemiological studies should be conducted occasionally to check the incidence rate of such chronic and disabling diseases.
Acknowledgement
The authors would like to express thanks for Prof. Dr. Shamim Akhter, Department of Zoology, PMAS Arid Agriculture University Rawalpindi, Pakistan, for great help throughout the work, fruitful guidance, brilliant and creative remarks that helped this modest work to be produced in this form.
Conflict of Interest
There is no conflict of interest.
References
Abildgaard, N., Petrsland, N., 1991. Hepatitis C virus transmitted by tattooing needles. Lancet, 2: 460-2.
Ali, H.M., Bhatti, S., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., Muhammad, A., 2015. Mutational analysis of MDM2 gene in hepatocellular carcinoma. Sci. Lett., 3(1):33-36.
Dubois, F., Francios, M., Mrriotte, N., 1997. Serum alanine aminotransferase measurement as a guide to selective testing for hepatitis C during medical checkup. J. Hepatol., 112: 463-72.
Dufour, D.R., Loot, J.A., Nolte, F.S., Gretch, D.R., Koff, R.S., Scoff, B.C., 2001. Diagnosis and monitoring of hepatitis injury. J. Clin. Chem., 46(12): 2027-49.
Dushaiko, G.M., 1990. Progress in hepatitis C research. Lancet, 334: 605-6.
Fattovich, G., Dally, G., Bahr, M.J., 1997. Morbidity and mortality in compensated cirrhosis type C. Gastoenterol., 112: 463-72.
Haurigan, K.J., Bowling, F.G., 2001. Alcoholic liver disease. J. Gastroenterol. Hepatol., 16: 1138-43.
CrossRef | Google Scholar
Hollinger, F.B., 1990. Non-A and Non-B hepatitis viruses. J. Virol., 12(7): 1394-98.
CrossRef | Google Scholar
Hoofnagle, J.H., Bisceglie, A.M., 1998. The treatment of chronic hepatitis. N. Engl. J. Med., 336(5): 347-55.
CrossRef | Google Scholar
Kiyosawa, k., Sodenyma, T., Tanaka, E., 1991. Hepatitis C virus in hospital. Viral Hepatol., 8(4): 256-63.
CrossRef | Google Scholar
Loannou, G.N., Boyko, E.J., Lee, S.P., 2006. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am. J. Gastroeterol., 101(1): 76-82.
CrossRef | Google Scholar
Marengo, R.A., 1993. Hepatitis C as an update. The Doctor, 25(4): 6-8.
CrossRef | Google Scholar
Muhammad, A., Farooq, M.U., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., 2013. Prevalence of diabetes mellitus type II in patients with hepatitis C and association with other risk factors. Punjab Univ. J. Zool., 28 (2): 69-75.
CrossRef | Google Scholar
Pereia, B., Milford, E.L., Krlkman, R.L., 1991. Transmission of hepatitis C virus by organ transplantation. N. Engl. J. Med., 325: 454-60.
CrossRef | Google Scholar
Prince, M., Chetwynd, A., Newman, W., Medcalf, J.V., James, O.F.W., 2002. Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis. Gastroenterol., 123: 1044-51.
CrossRef | Google Scholar
Schafer, D.F., Sorrell, M.F., 1997. Liver failure. N. Engl. J. Med., 336(16): 1173-74.
CrossRef | Google Scholar
Schalm, S.N., Fattovich, G., Bronwer, J.T., 1997. Therapy of hepatitis C patients with cirrhosis. Hepatol., 26(3): 1285-1325.
CrossRef | Google Scholar
.Seng, L.T., Katzel, G.M., 2001. How hepatitis C virus counteracts the interferon responses. Virol., 285: 1-12.
CrossRef | Google Scholar
Stanley, A.J., Haydon, G.H., Piris, J., 1996. Assessment of liver histology in patients with hepatitis C and normal transaminase levels. Eur. J. Gastroessnterol. Hepatol., 8(9): 869-72.
CrossRef | Google Scholar
Tarao, K., Rino, Y., Ohkawa, S., Tamai, S., Miakawa, K., Takakura, H., Endo, O., Yoshitsugu, M., Watanabe, N., Matsuzaki, S., 2002. Close association between high serum alanine aminotransferase levels and multicentric hepatocarcinogenesis in patients with hepatitis C virus-associated cirrhosis. Cancer, 94(6):1787-95.
CrossRef | Google Scholar





