Antimicrobial Activity of Cinnamon Barks (Aqueous and Ethanolic Extracts) and some Antifungals against Candida albicans Isolated from Oral Candidiasis in Leukemia Patients
*Corresponding author:
Entisar Esmail Al-Hakami;Email:
alhakamie@gmail.comViews 2836
Abstract
Oral pharyngeal candidiasis (Oropharyngeal Candidiasis) is an opportunistic disease resulting from weakness of immune system caused by chemotherapy to Leukemia patients. Candida albicans yeast is the causative agent of this infection. There are many types of antifungals used to treat this fungal infection depending on the degree or type of candidiasis; but noted in recent days, increasing resistance to antifungals. This study included isolation and identification of Candida from oral cavity of ten leukemia patients who suffered from oral candidiasis in three hospitals in Sana’a city. Samples were collected by swabbing the lesional tissues in the oral cavity and cultured on Sabroud’s Dextrose Agar SDA. Six of the oral swabs gave noticeable growth on this medium, while 4 of them didn’t give any growth. Morphological and biochemical tests done to the six isolates confirmed that all were Candida albicans. These isolates were tested for antifungal susceptibility test. Antifungal agents used in this test were Miconazole 50µg, Fluconazole 25µg, and Nystatin 100µg. Results showed that Miconazole was the most efficient antifungal against all tested isolates, while Nystain and Fluconazle showed various inhibitory effects among tested isolates. Some isolates were resistant, while others were susceptible. Due to resistance development of some Candida isolates to antifungal agents, Cinnamon was examined for its ability to inhibit the growth of this yeast. Cinnamon aqueous and ethanolic extracts were prepared by using bioshaker. Antimicrobial activity of cinnamon extracts were screened by using agar disk diffusion technique ADD, and tested C.albicans inoculums were prepared by bioassay plate method. The results showed that cinnamon aqueous extract 100% concentration could slightly inhibit the growth of some isolates. By contrast cinnamon ethanolic extract could effectively inhibit the growth of all tested C.albicans isolates. The Minimum Inhibitory Concentration MIC of ethanolic extract was varied among C.albicans isolates.
Keywords
Leukemia, Oropharyngeal Candidiasis, Cinnamon aqueous and ethanolic extracts, Antimicrobial Activity.
Citation
Al-Hakami, E.E., Al-Kassari, O.A., Al-Helali, M.F., 2016. Antimicrobial Activity of Cinnamon Barks (Aqueous and Ethanolic Extracts) and some Antifungals against Candida albicans Isolated from Oral Candidiasis in Leukemia Patients. PSM Microbiol., 01(1): 18-25.
Introduction
The word Candida coins from the Latin word candid, meaning white (Zunt, 2000). Yeast (genus Candida) comprises 150-200 species (Odds,1988), imperfect unicellular dimorphic fungi, reproduce by budding, form hyphae and /or psuedohyphae (Richardson and Warnode, 2003).They were consigned to the family deutromycetes , representing a deficiency of sexual reproduction (Chalderon, 2002).
Candida albicans species is accompanying with typical oral carriage in humans, which are taking place in the mouths of healthy individuals (upto 80%) (Odds, 1988). The pathogenic state of Candida can occur due to changes of the oral cavity according to the environment (favorable for Candida growth), and these changes are influencing aspects for Candida infection (candidiosis) and in severe cases responsible for faintness of host immune defenses (Williams and Lewis, 2011).
Elevation of liver enzymes is responsible for liver disorders (Toor et al., 2016). It has been demonstrated that age, sex, diet, alcohol, and infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV) as the risk factors associated with Hepatocellular carcinoma (Ali et al., 2014). Hepatitis C virus HCV infection is also linked with diabetes mellitus, with hepatitis B virus (HBV) infection, hypertension and angina pectoris (Muhammad et al., 2013; Iqbal et al., 2016). Liver disorders lead to development of various types of cancer.
Undergoing chemotherapy Leukemia patient shows best condition for the expansion of oral candidosis, particularly in the course of neutropenia and these species causes almost one half oral infections taking place for the duration of antileukemia therapy (Dreizen, 1990). A significant number of deaths among leukemia patients are due to Candida septicemia, some of these are accompanying by means of previous oral infection (Sinnett et al., 1992).
Treatment of the infections caused by candida is the usage of antifungal mediators i.e., azoles (fluconazole, itraconazole, miconazole and ketoconazole) and polyenes (amphotericin B or nystatin). There are many problems in controlling these infections, such as inadequate number of effective antifungal mediators, their high poisonousness and expenditures, the reappearance of the infection and the most important one is the growing resistance to them (Klesper,2001; Khan et al.,2003).Therefore, natural substances particularly derivative from the plant species are gaining lot of significance because of their obtainability and widely usage and assurance of safety.
Previous Research (in-vitro and in-vivo) in different organisms (animal and human) from various portions of the world have established several advantageous fitness properties of cinnamon, such as anti-microbial activity, decreasing cardiovascular disease, increasing intellectual function, anti-inflammatory properties and decreasing danger of colonic cancer (Jayaprakasha and Rao, 2001).
Hence, the present study was designed to extract the cinnamon in aqueous and ethanolic state and study the effect of cinnamon extracts against some isolates of Candida albicans obtained from oral cavity of leukemia patients suffering from oral candidiasis, and compared with some antifungal agents.
Materials and Methods
Collection of samples
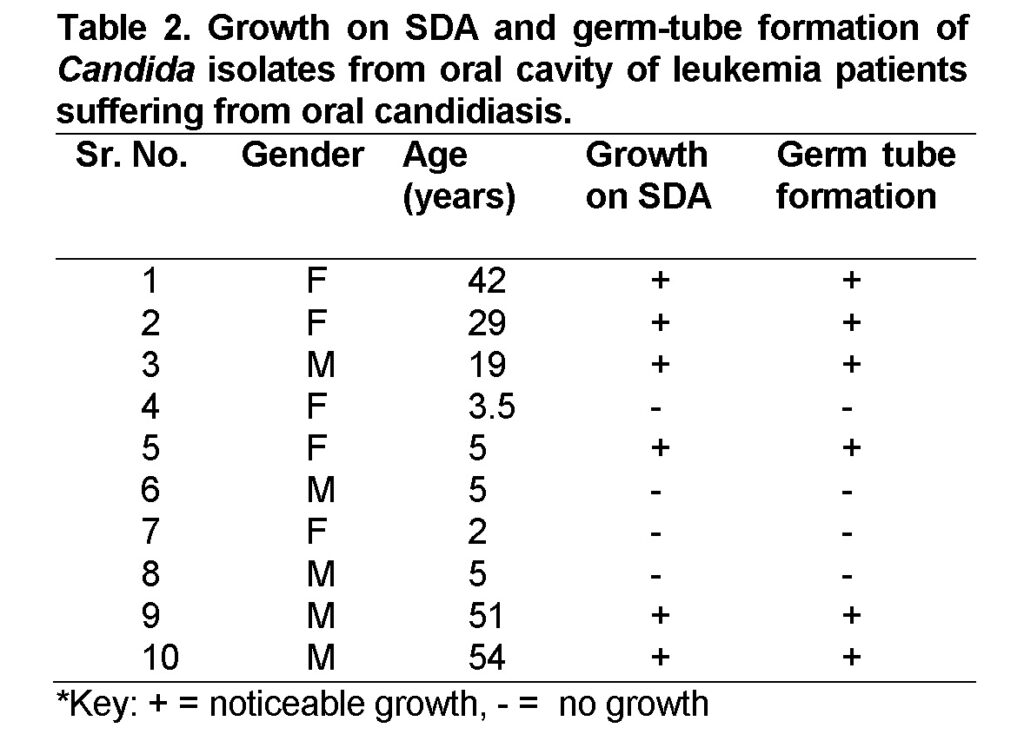
The samples were collected from 10 leukemia patients suffering from oral candidiasis from three hospitals in Sana’a city (Al-Thawra hospital, Al-Gomhore hospital, and Al-Kuwait University hospital) (Table 1).
Isolation of Candida albicans
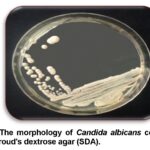
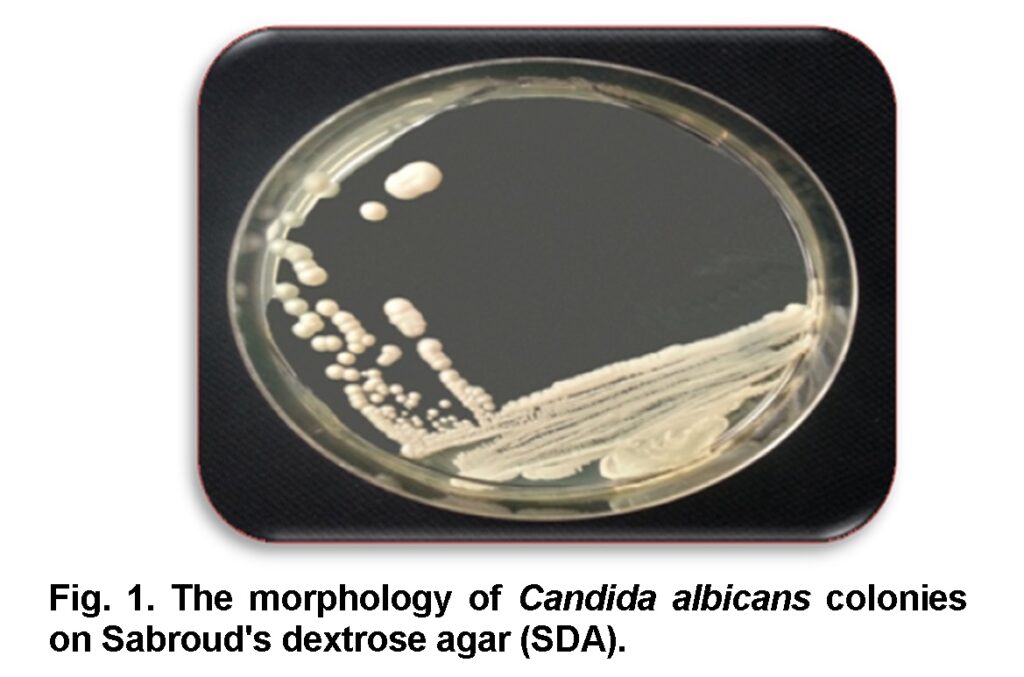
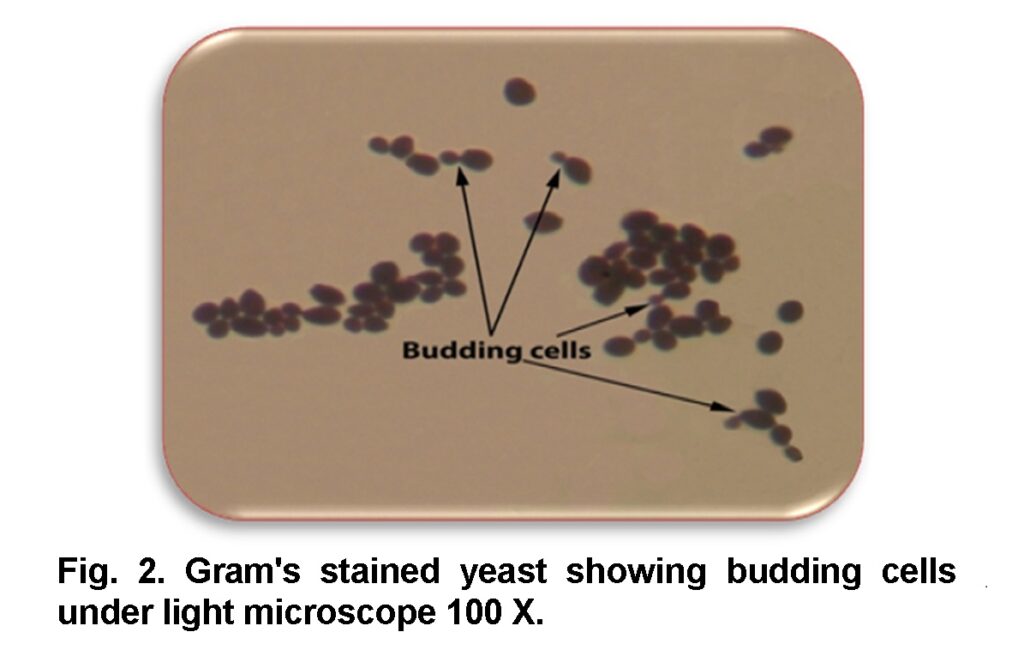
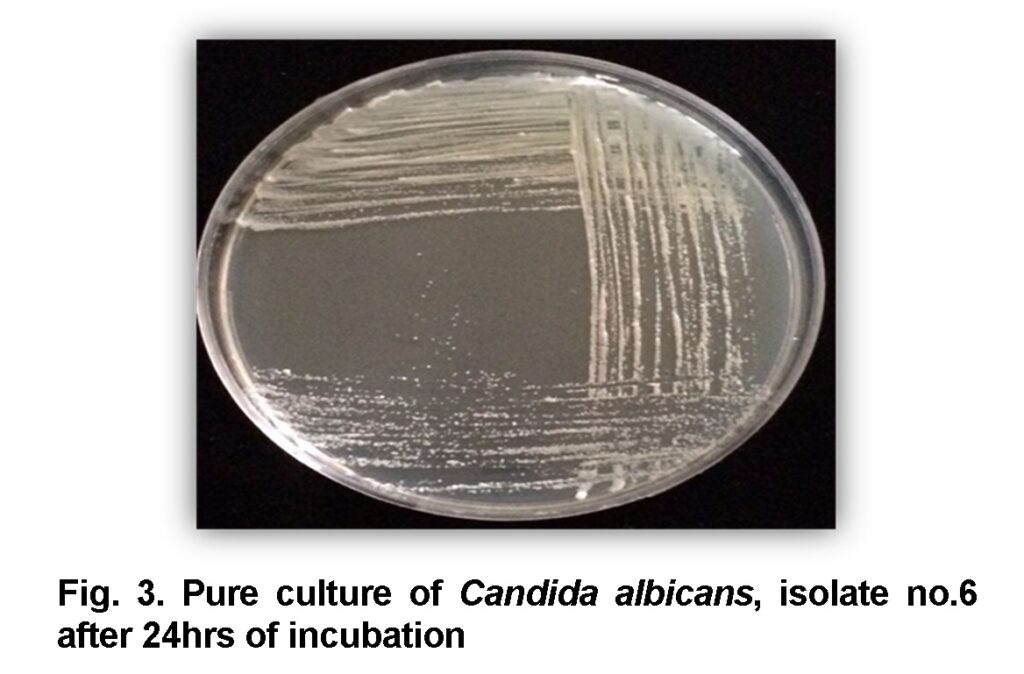
The obtained samples were consequently inoculated to the medium Sabouraud’s dextrose agar (SDA) (Axéll et al., 1985) and placed in an incubator at 37°C for 24 to 48 hrs. Cream, smooth, pasty, convex colonies of candida on SDA were developed after 24 hrs (Figure 1) (Baveja, 2010). A single colony was transferred by sterilized loop to a clean slide containing a drop of tap water to examine under light microscope. Candida is a yeast so it can be identified by its oval shape and budding cells. To make the slide clearer the slide was stained by Gram’s stain (Figure 2). After observing the budding cells, a small amount of the same colony was subcultured and streaked on Petri dishes and agar slants containing SDA and placed in an incubator at 37°C for 24 hrs to make a pure culture (Figure 3, 4) for the second step.

Germ -tube test
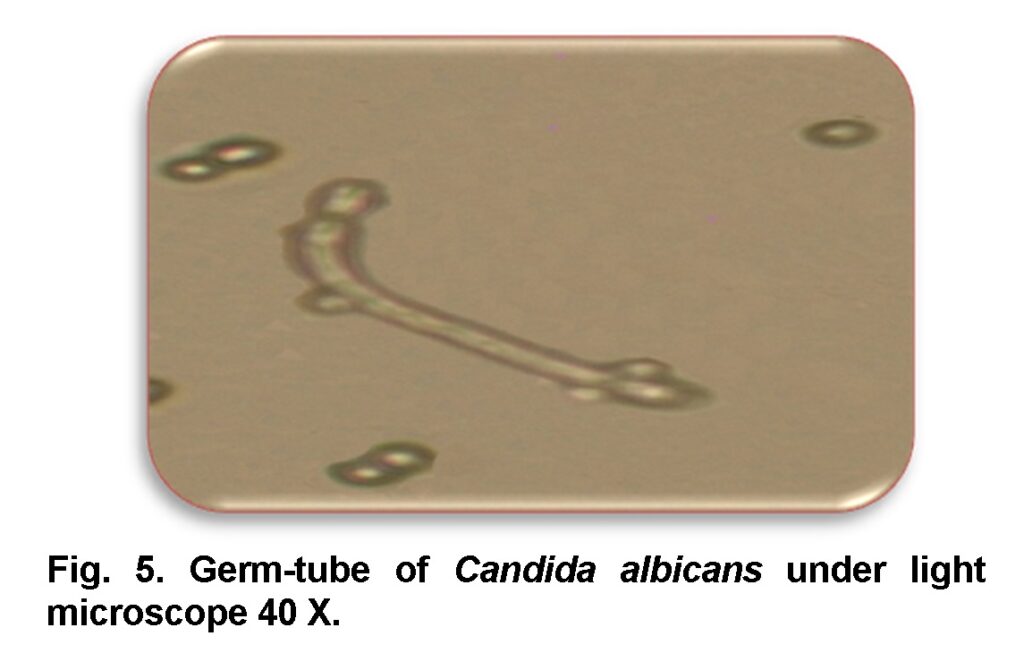
The growing strain was then subjected to germ tube test for identification of C.albicans by using the method of Williams and Lewis, 2000. At room temperature, cells were put off in human serum (0.3-0.5) ml and then incubated at 37ºC for 2 to 4 hrs. After incubation; one or two drops of culture was positioned on a clean glass slide and observed under microscope (Figure 5).
Preparation of Cinnamon Extracts
Dry cinnamon were obtained from local retail market (Yassin for spices) in Sana’a city, Yemen, and then brought to the Laboratory and crushed by means of a grinder into a fine powder.
Ethanol Extract
100g of cinnamon powder was saturated in 400ml of Ethanol in sterilized flask 1000ml with constant agitation (130 rpm) whole night in the bio shaker (at 20°C). The ethanol was isolated utilizing decontaminated cheesecloth and filtered through sanitized Whatman filter paper (No.2). After that the extract was left to dry at 40°C in hot air oven for evaporation of ethanol.
Aqueous Extract
100g of cinnamon powder was drenched in 400 ml of sterilized distilled water in sterilized flask with constant agitation (130 rpm) whole night in the bio shaker (at 20°C). The aqueous portion was extracted utilizing decontaminated cheesecloth and filtered through sanitized Whatman filter paper (No.2). After that the extract was left to dry at 40°C in hot air oven for evaporation of water. Both ethanol and aqueous extracts were kept in small sterilized plastic bottles (these were kept in Petri dishes which covered by foil paper) at (-20°C) until use (Hoque et al., 2008).
Preparation of Cinnamon extracts dilutions
At the beginning both aqueous and ethanolic extract were prepared at 100% concentration by adding 1 g of the extract into 1 ml of the solvent DMSO. After reading the results ethanolic exract was diluted by DMSO to investigate the minimum inhibitory concentration MIC. Various dilutions of ethanolic extract were prepared as 100%, 80%, 60%, 40%, 20%, 15%, 10%, and 5%.
Screening for antimicrobial activity
Inoculums Preparation
A loopful of each Candida albicans isolates were transferred individually from agar slants into tubes containing 4 to 5 ml of decontaminated brain heart infusion broth (BHIB). The cultures broth was then placed in an incubator for 18-24 hrs at 37°C. After incubation period amount of these fresh cultures were transferred by sterile syringe into another tube containing sterile normal saline for turbidity which is analogous to McFarland standard. As a result almost 1 to 2×108 CFU/ ml Candida albicans was obtained in a suspension.
Inoculation of Candida albicans inoculums into the culture medium
The culture medium used in this test was SDA. For quantitative analysis of Candida suspension for the purpose of bioassay plates following formula was used.
X=TV/C
(Where: X= amount of stock culture suspension to be added to the melted agar, T= target (desired) number of candida cells in lawn, V= volume of molten agar (ml). C= conc. of stock culture suspension). The required volume of the cell suspension was added to the melted agar that cooled until 45°C. Agar solutions were mixed by swirling using sterile syringe. Then 20 ml of inoculated liquid agar were poured into 90ml sterile Petri dishes with tilt to make the agar uniformly covers the plate and it was made sure that there is no holes or bubbles. Then let the agar to harden in a flat, level surface (Laboratory Guide Book, 2007).
Add cinnamon extracts to bioassay plates
In this study agar disk diffusion method was used. Filter paper discs of 6mm diameter using Whatman no.1 were prepared and sterilized. It has soaking in the spice extracts. Triplicate disks of each extract and dilution was placed in the center of the appropriate section on the medium. Then the plates were placed in refrigerator for 1/2 to 1 hour for appropriate diffusion and after that the plates were placed in an incubator (upright position) for 24 hrs at 37°C. The zones of inhibition were then measured by using ruler and recorded in millimeters to determine the antimicrobial activity of each concentration and extract. Then the mean values of the diameter of zones of inhibition were determined.
Antifungal susceptibility test
The steps of the preparation of C.albicans inoculums and agar plates were explained previously. Disk diffusion test for Miconzole 50µg, fluconazole 25µg, and Nystatin 100µg was performed. Antifungals disks were fixed in an appropriate section with consideration that the space between antifungal disks is equal. Then the plates were placed in refrigerator for 1/2 to 1 hour for appropriate diffusion and after that the plates were placed in an incubator (upright position) for 24 hrs at 37°C. The zones of inhibition were measured by using ruler and recorded in millimeters to determine the antimicrobial activity of each antifungal.
Results and Discussion
A total of 6 isolates of Candida albicans were purified from oral cavity of ten leukemia patients suffering from oral candidiasis. The results showed that six of the ten oral swabs gave noticeable and significant growth on Sabouraud’s dextrose agar SDA, while four of them didn’t give any growth on the same medium (Table 2). These four swabs were from leukemia patients aged between 2 to 5 years. So the negative results of these swabs maybe due to mistakes during sampling. The morphology of colonies on SDA of the six isolates were cream, smooth, pasty, and convex. Microscopic examination of the isolates showed budding cells that identify yeasts. Germ-tube test showed that all of the positive swabs were Candida albicans, that is approves with previous studies of Odds,1988; Sinnett et al., 1992; Raju and Rajappa, 2011who stated that C.albicans as the most frequently cultured Candida species from oral cavity.
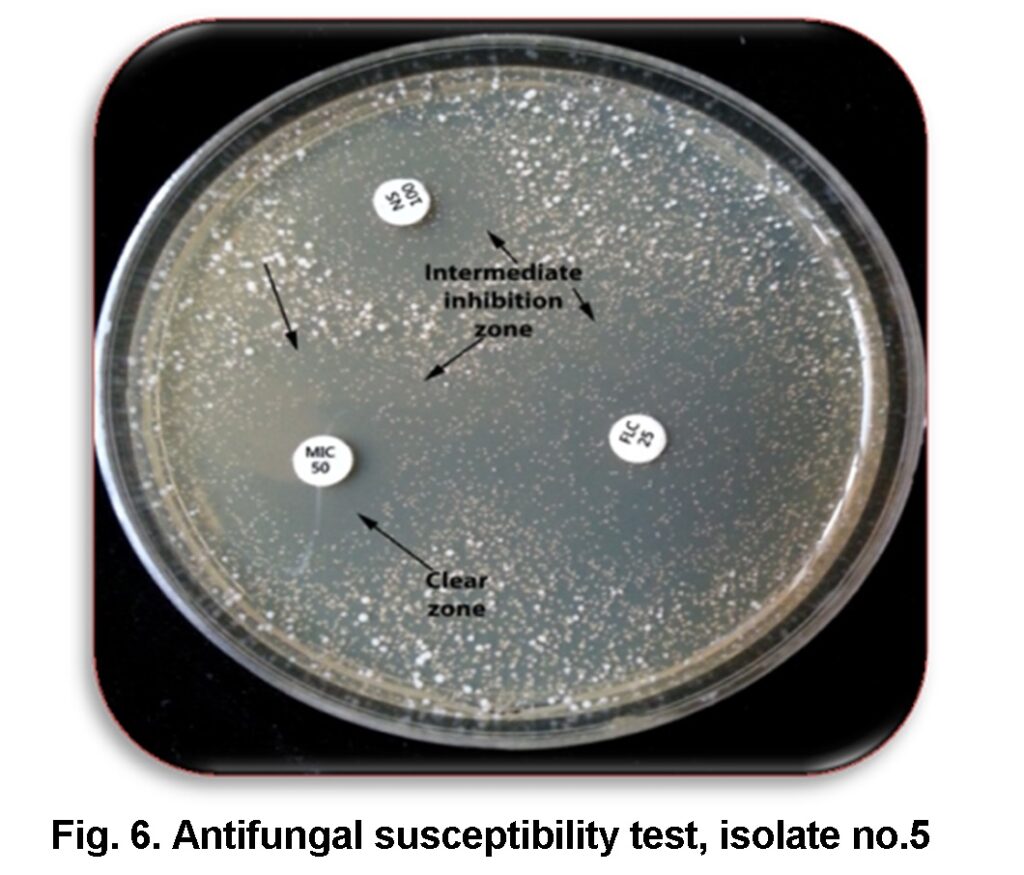
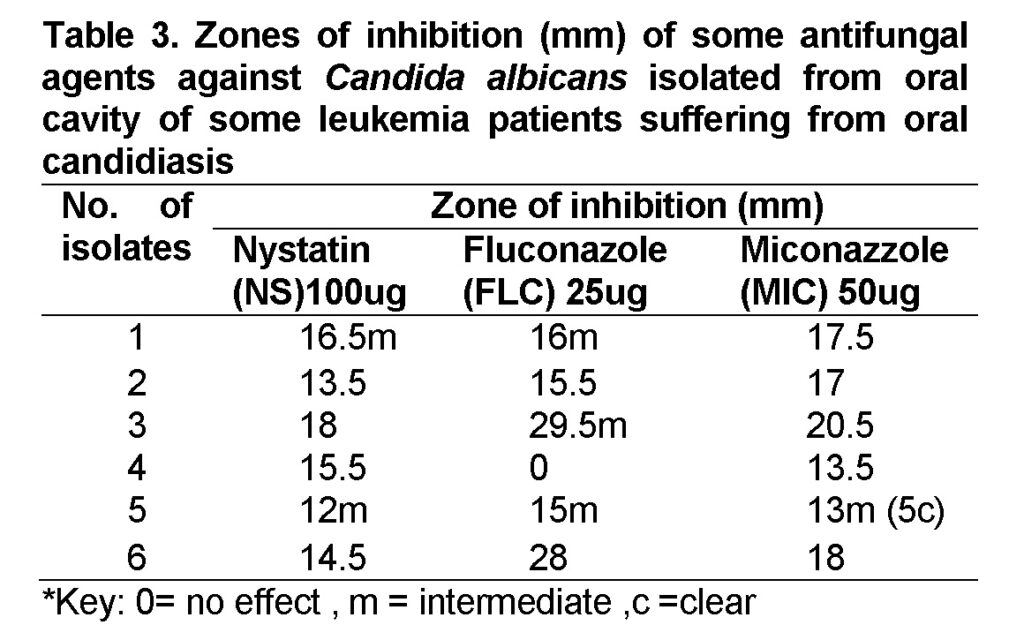
Our results illustrated that C.albicans isolates showed various sensitivity to antifungal agents (Table 3). Results showed that miconazole 50µg could effectively inhibit the growth of all isolates with clear zone, except isolate no.5 which showed intermediate inhibition zone 13mm and clear inhibition zone 5mm (Figure 6). This means that none of the isolate was resistant to miconazole. Similar results were also obtained by Carvalinho and Costa (2012) who did not find any isolate resistant to this antifungal.
Fluconazole 25µg could effectively inhibit the growth of only one isolate (isolate no.2), and could inhibit ineffectively the growth of isolates no. (1, 3, 5 and 6) with intermediate inhibition zone (16mm, 29.5mm, 15mm, 28 mm) on following. Isolate no.4 was resistant to this antifungal. However,(Kuriyama et al., 2005) and (Dorocka-Bobkowsha et al., 2007) described that 88.7% of C. albicans were fluconazole susceptible. These results documented that fluconazole have efficient anticandidal activity. Nevertheless the results that we obtained in this study demonstrated that fluconazole has inefficient anticandidal activity against some isolates. Our results showed that C.albicans isolates may have developed resistance to this antifungal.
Nystatin 25µg could effectively inhibit the growth of isolate no.3 with inhibition zone 18mm. In addition this antifungal could inhibit the growth of isolate no.2 and no.4 with inhibition zone 13.5mm, 15.5mm on following. While it could ineffectively inhibit the growth of isolates no.1, no.5 and no.6 with inhibition zone 16.5mm, 12mm, 14.5mm on following. By contrast, (Carvalinho and Costa 2012) found out that Nystain was efficient against all tested C.albicans. So our results maybe because Nystatin imported to Yemen is not effective in killing oral C.albicans completely or maybe C.albicans isolates developed resistance to this antifungal.
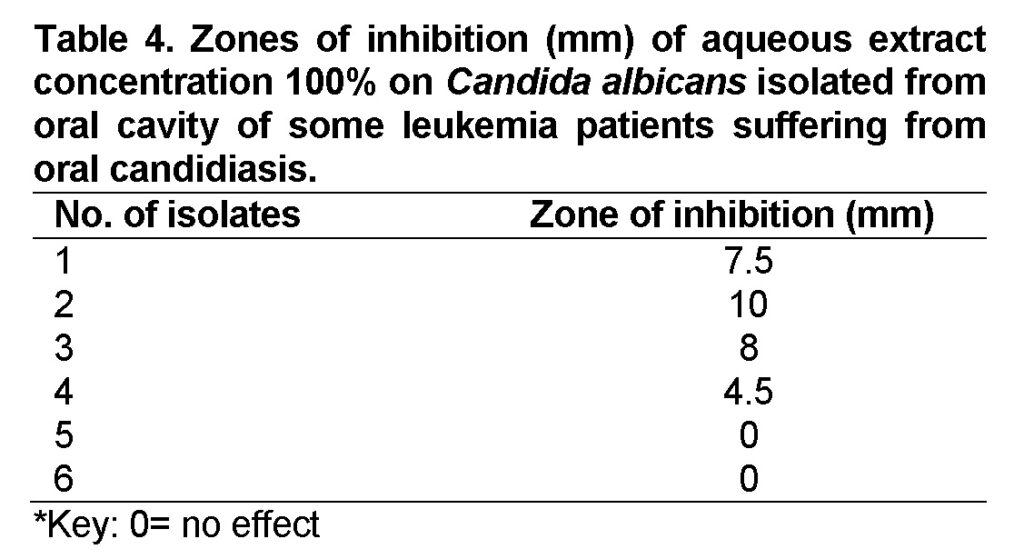
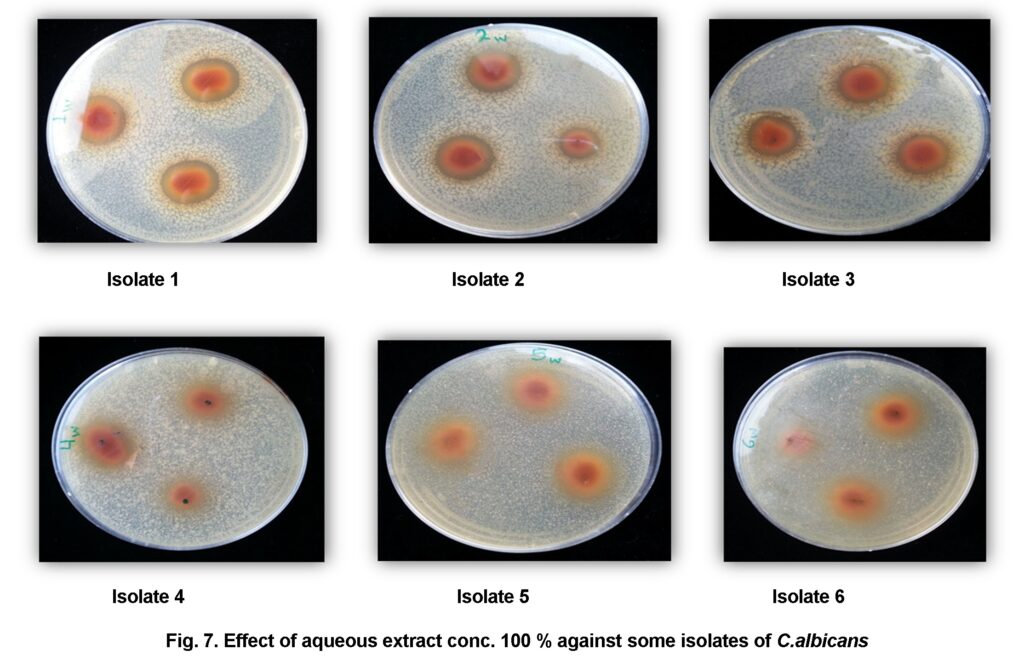
The antimicrobial activity of cinnamon extracts studied against the tested Candida albicans isolates by agar disk diffusion are shown in (Table 4 and 5).The results showed that aqueous extract 100% concentration could slightly inhibit the growth of some isolates (isolates no.1, no.2, no.3, and no.4) with inhibition zone (7.5mm, 10mm, 8mm, 4.5mm) respectively. Nevertheless it couldn’t inhibit the growth of isolates no.5 and no.6 (Figure 7).
According to our results ethanolic extract showed various inhibitory effects against C.albicans isolates. One hundred percent concentration inhibited the growth of all isolates with large and clear inhibition zones except isolate no.5 and 6 which showed large but intermediate inhibition zone.
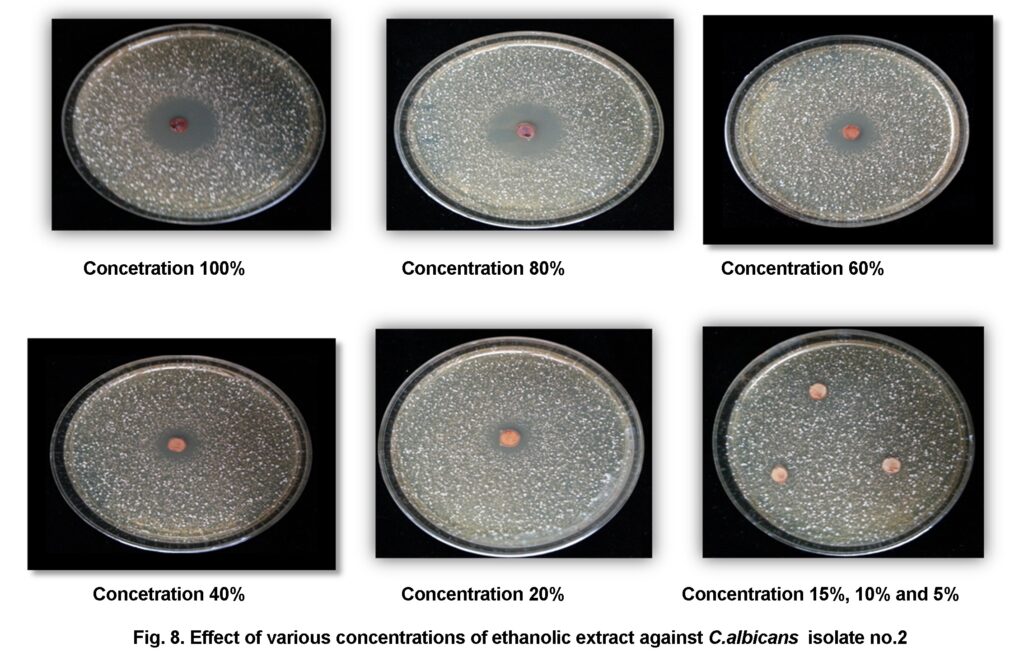
The results of MIC showed increase of growth with decrease of concentration and the MIC was various among C.albicans isolates. The MIC of Cinnamon ethanolic extract on isolates (no.1, no.2, no.3, and no.5) was 5% and the inhibition zones of this concentration on this isolates were (3.5 mm, 3.5 mm, 5.5 mm, 5.5 mm) on following. While the MIC of the same extract on isolate no.4 was 15% with intermediate inhibition zone 2.5mm. Finally the MIC of ethanolic extract on isolate no.6 was 20% with inhibition zone 14.2mm (Figure 8).
In general, cinnamon ethanaolic extract showed the highest anticandidal activity against C.albicans isolates under study (mean of inhibition was 26mm). While cinnamon aqueous extract showed the lowest anticandidal activity (mean of inhibition was 3.5mm) (Table 6). Similar results were obtained from previous studies. (Pfaller., 2000) studied the influence of spices on the growth of C.albicans by using agar disk diffusion assay technique. The researchers observed that the concentrated cinnamon extract could slightly prevent the growth of Candida albicans. The diameter of zone inhibition produced by boiled concentrated extract was 9mm. On the other hand, the diameter of zone inhibition produced by boiled diluted extract was 0mm.
Among C.albicans isolates, isolate no.3 was the most affected by the anticandidal agents under study while isolate no.5 was the lowest affected by the same anticandidal agents.
Conclusion
The results of this work suggest that the compounds extracted from Cinnamon have a broad spectrum of antimicrobial activity and this effect is increased by increasing the quantity of this compound, which can be used as an alternative for antifungals. Therefore, pharmacological test is necessary to isolate and characterize it’s active compounds. Moreover, cinnamon extracts should be investigated in vivo to better understand their safety, efficacy and properties.
Recommendations
Oral hygiene and topical antifungals are usually adequate for uncomplicated oral candidiasis. Oral hygiene involves cleaning the teeth, buccal cavity, tongue, and dentures, if present, daily. Culture and sensitivity testing should be undertaken if initial therapy is unsuccessful. To use cinnamon active compounds as alternative to chemical antifungal agents. Don’t to use cinnamon directly to lesional tissues in oral cavity, because cinnamon constituents may be irritating to the oral mucous membranes.
Acknowledgement
Authors are very grateful to Professor Mohammed F. Al-Helali, Microbiology Section, Biology Department – Faculty of Science, Sana’a University, Yemen for technical assistance.
Author’s Contribution
Entisar Esmail Al-Hakami and Omima Ali Al-Kassari collected samples, performed experimental work and write manuscrip; Mohammed F. Al-Helali proofread and approved the final manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Ali, H.M., Bhatti, S., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., Muhammad, A., 2015. Mutational analysis of MDM2 gene in hepatocellular carcinoma. Sci. Lett., 3(1):33-36.
Axell, T., Simonsson, T., Birkhed, D., Rosenborg, J., and Edwardsson, S., 1985. Evalution of a simplified diagnostic aid (Oricult-N) for detection of oral candidosis . Scand J Dent Res., 93(1): 52-55.
Baveja, C., 2010. Medical Mycology, Text Book of Microbiology for Dental Students,Arya Puplications , Delhi , India, 3rd edition., 322-323.
Carvalhinto, S., Costa, A.M., Coelho, A.C., Martins, E., Sampaio, A., 2012. Susceptibilities of Candida albicans mouth isolates to antifungal agents, Essentials oils and mouth rinses. Mycopathol., 174:69-76.
Chalderon, A.R., 2002. Candida and Candidiosis .4th Edition (ASM press; Washington).chap2, pp. 15- 27.
Dorocka-Bobkowska, B., Konopka, K., 2007. Susceptibility of Candida isolates from denture-related stomatitis to antifungal agents in vitro. Int J Prosthodont., 20: 504–6.
Dreizen, S., 1990. Description and incidence of oral complications. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health. Consensus Development Conference on Oral Complications of Cancer Therapies: Diagnosis, Prevention, and Treatment. NCI. Monographs., 9: 11-15.
Pfaller, M.A., 2000. Antifungal susceptibility testing: progress and future developments. Braz J Infect Dis. 4(2): 55-60.
Hoque, M., Bari, M. L., Juneja, K.V. , and Kawamoto, S. 2008. Antimicrobial Activity of Cloves and Cinnamon Extracts against Food Borne Pathogens and Spoilage Bacteria , and Inactivation of Listeria monocytogenes in Ground Chicken Meat with their Essential Oils .Rep. Nat l. Food Res. Inst ., 72: 9-21.
Iqbal, M.N., Ashraf, A., Yunus, F.N., Muhammad, A., Alam, S., Xioa, S., Ali, S., Irfan, M., 2016. Prevalence of Angina Pectoris in relation to various risk factors. PSM Biol. Res.,01(1): 06-10.
Jayaprakasha, G.K., Rao, L.J., Sakariah, K.K., 2002. Chemical composition of volatile oil from Cinnamomum zeylanicum Buds. Z. Naturforsch. C., 57: 990-993.
Khan, A., Safdar, M., AliKhan, M. M., Khattak, K.N. , Anderson, R.A., 2003. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care., 26(12): 3215-8.
Klesper, M.E., 2001. Antifungal resistance among Candida species. Pharmacother., 21: 124S-32S.
Kuriyama, T., Williams, D.W., Bagg, J., Coulter, W.A., Ready, D., Lewis, M.A., 2005. In vitro susceptibility of oral Candida to seven antifungal agents. Oral Microbiol Immunol., 20:349–53.
Laboratory Guide Book Notice Change., 2007. Bioassay for the Detection, Identification and Quantitation of Antimicrobial Residues in Meat and Poultry Tissue. United States Department of Agriculture Food Safety And Inspection Service, Office of Public Health Science.1-60. MLG 34.02.
Muhammad, A., Farooq, M.U., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., 2013. Prevalence of diabetes mellitus type II in patients with hepatitis C and association with other risk factors. Punjab Univ. J. Zool., 28 (2): 69-75.
Odds, F.C., 1988. Candida and candidiasis. 2nd Ed. London: Bailliere Tindall., 468.
Raju, S.B., Rajappa, S., 2011. Isolation and Identification of Candida from the Oral Cavity. ISRN Dent., Volume 2011, Article ID 487921, 7 pages. http://dx.doi.org/10.5402/2011/487921.
Richardson, M.D., Warnock, D.W., 2003. Fungal Infection: Diagnosis and Management, 3rd Edition. Blackwell Publishing. ISBN 0-443-07937-4.
Sinnett, A.E. , Childers, K.N. , Wright, T.J. , Rodu, K.B., Edwin, L. ,and Baradely, J.r., 1992: The detection of oral Candida in pediatric leukemia patients. Pediatric Dentistry., 14(4): 236-239.
Toor, S., Toor, S., Ashraf, A., Alam, S., Anwaar, S., Saddiqa, A., Ali, S., Muhammad, A., Toor, S., Akhter, S., 2016. Prevalence of Liver disorders in Islamabad City. PSM Biol. Res., 01(1): 31-33.
Williams, D. and Lewis, M., 2011. Pathogenesis and Treatment of Oral Candidosis . J Oral Microbiol., 3:10.3402/jom.v3i0.5771. doi: 10.3402/jom.v3i0.5771
Williams, D.W. and Lewis, M.A.O., 2000. Isolation and identification of Candida from the oral cavity. Oral Diseases., 6(1): 3-11.
Zunt, S.L., 2000. Oral Candidiasis: diagnosis and treatment. JPH., 9: 31-36.