Screening of Soil Isolates of Bacteria for Antagonistic Activity against Plant Pathogenic Fungi
*Corresponding author:
Fatma Ahmed Ali Alsohiby;Email:
ahmedalialsohiby@gmail.comViews 3027
Abstract
The most common approach to biological control comprised of selecting antagonistic microorganisms, studying their modes of action and establishing a biological control product. The present study was an attempt to screening of soil isolates of bacteria for antagonistic activity against plant pathogenic fungi. Bacterial isolates from soil samples were identified by cultural, morphological and biochemical characterisation. Bacterial isolates were then screened for their antagonistic activities against the tested plant pathogenic fungi. A total of 14 isolates belonging to 4 different species of bacteria with inhibitory activity against selected fungi were isolated from soil samples and identified biochemically. They were recognised in the genera Bacillus (B. subtilis, and B. amyloliquefaciens), Klebsila spp. and Micrococcus spp. The effect of the antagonistic activity of Klebsila sp, Bacillus sp and Micrococcus sp against plant pathogenic fungi was checked. The average diameter of inhibition zone (cm) of bacterial isolates against plant pathogenic fungi Penicillium purpurogenum was recorded as Klebsila spp. 2cm, Bacillus subtilis 2.6cm, B. amyloliquefaciens 0.866cm and Micrococcus spp. 1.6cm. With respect to the considerable tolerance of B. subtilis to environmental stresses and their facile production by current fermentation technology, bacterial isolates identified in this study with a diverse range of antifungal activities can be known as prospective resources of novel naturally occurring antifungal agents for controlling pathogenic fungi in medicine and agriculture. Biological control practices require an integrative approach, and more knowledge than chemical control.
Keywords
Biological control, antagonistic microorganisms, plant pathogenic fungi, inhibition zone.
Citation
Alsohiby, F.A.A., Yahya, S., Humaid, A.A., 2016. Screening of Soil Isolates of Bacteria for Antagonistic Activity against Plant Pathogenic Fungi. PSM Microbiol., 01(1): 05-09.
Introduction
The fungal kingdom consists of an estimated 1.5 million species on our planet. Among nearly 100,000 described species of fungi, approximately 400 species is now recognized as pathogens to humans, animals and plants. They include both molds and yeasts from different genera and species with the majority belonging to the genera Fusarium spp., Alternaria chlamydospora (Samson et al., 2000).
The fungi belonging to the genera Fusarium spp., Alternaria chlamydospora are important for not only causing life-threatening infections in humans and animals, but also producing toxic metabolites named “mycotoxins”. Fusarium spp.is an important plant pathogenic fungus capable of producing different mycotoxins in food and agricultural commodities (Gallo et al., 2015).
Effective control of plant pathogenic fungi involves different methods such as: cultural control, resistant cultivars and chemical control. Toxic compounds (potentially hazardous to humans and the environment) are accumulated by the rigorous usage of fungicides and also responsible for the resistance of the pathogens. In interpretation of these phenomenon and the presentation of biological control mediators (BCAs) appears to be one of the auspicious methods (Zivkovic et al., 2010). Synthetic chemicals including antifungal drugs and fungicides are widely used to handle injurious properties of fungi on human health and agriculture. Although fungicides are a key component of disease management programs, they grieve after large restrictions containing antagonistic responses on biological systems, resistance expansion of fungal pathogens and unwanted possessions proceeding non-target beneficial microorganisms. Thus, the optimization of environmentally- friendly fungicides is preferred (Ghisalberti, 2000).
The chief existing resources for antifungal identification are characterized by separating from the huge biodiversity ubiquitous in natural means (soil samples). The requirement for harmless and most functional agents (antifungal agents) for the pathogenic fungus has generated significant attention for screening of new agents by natural means (Ranjbariyan et al., 2011). Bio control can be induced by nonpathogenic naturally existing microorganisms which can interact with the pathogenic microorganism for food, constrain pathogen reproduction by discharging chemical agents (antibiotics or toxins) (Zivkovic et al., 2010).
Similarly, pathogenic fungal resistivity to presently obtainable antifungal agents such as antibiotics has promote challenged the shortage of novel antibiotics (antifungal agent). Bacteria are gaining more importance regarding their ability to produce a wide range of bioactive metabolites with antimicrobial capabilities. At the present time, antifungal compounds have been obtained on the large scale from bacteria. Many of the antifungal agents are coming up by the researchers yet. Subsequently, antifungal metabolites construction in microorganisms such as bacteria is moderately reliant on the strain and species, continuing examination for identification of new bacteria to rise novel antifungals discovery is presently completed all over the world (Ranjbariyan et al., 2011).
There is necessity to handle plant diseases, to sustain the worth and profusion of food, feed, and fiber formed by cultivators everywhere in the world. Various methods may be utilized to avoid, moderate or to control plant diseases. The present study was calculated to screen and separate potent bacteria from the rhizosphere soil samples for antimicrobial activity against plant pathogenic fungus.
Materials and Methods
Collection of samples
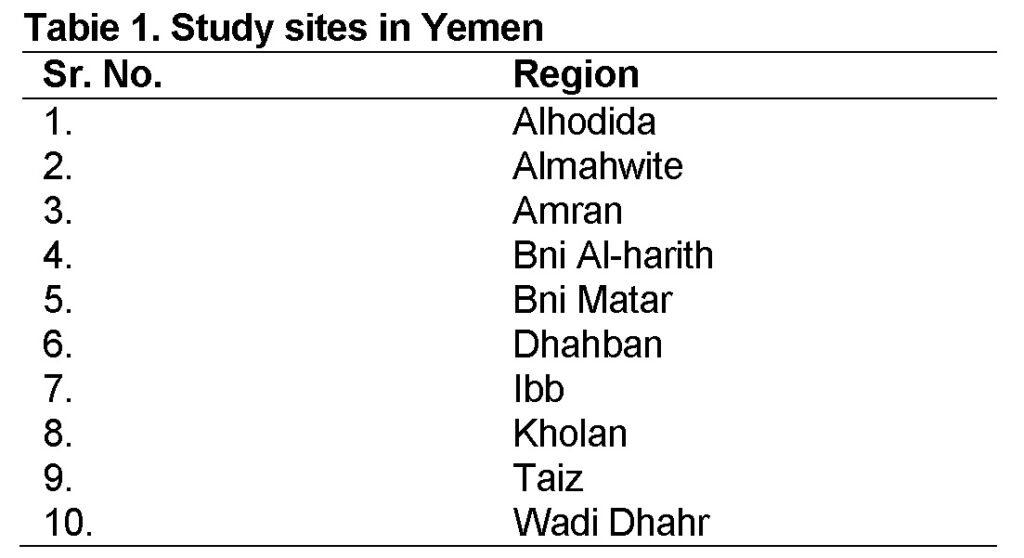
A total of 10 soil samples were collected 4 to 5 cm deep from the surface using spatula from different sites in Yemen (Table 1). The collected samples were kept in sterile polythene bags and sealed properly. The samples were transferred to Microbiology Laboratory, University of Sana’a Yemen and kept at 4ᵒC for further processing.
Tested fungal species
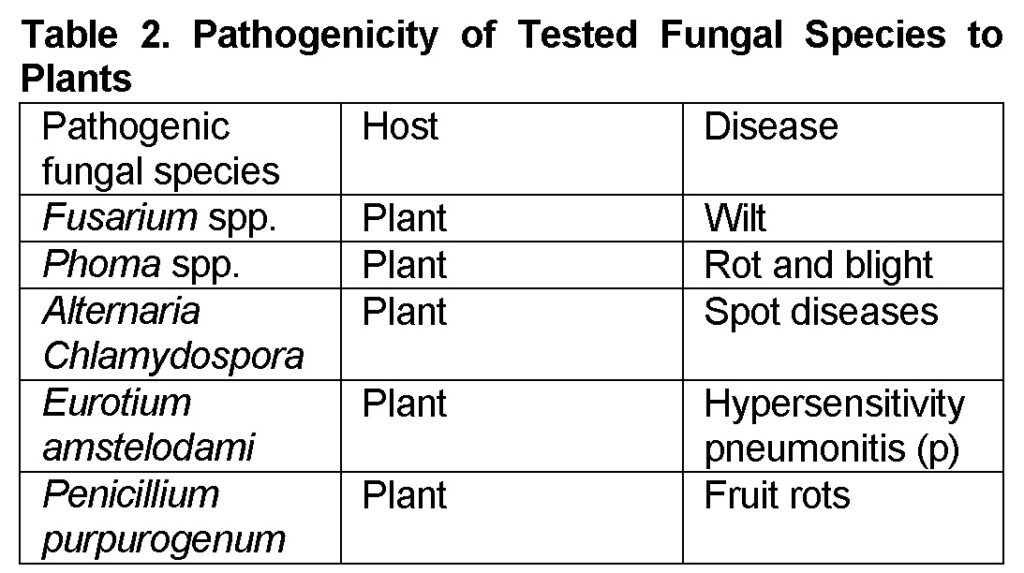
The fungal species were selected according to their Pathogenicity either to plant (Table 2) as recorded by (Lucas et al., 1992).
Isolation of bacteria from soil samples
Samples were processed by dissolving 1 g of soil in 10 ml of sterile distilled water to make soil suspension in test tube. The test tube was vortexed for 2 to 3 minutes to remove soil, stones and debris. Supernatant was transferred to another test tube and ten-fold serial dilutions were prepared. Then spread 100µl of supernatant from each dilution on nutrient agar plates and kept at 37ᵒC overnight.
After 24hrs the colonies were picked and streaked onto Nutrient Agar plates for purification. Pure colonies were transferred from these plates to Nutrient Agar slants, incubated at 37ᵒC for 24 hr and stored at 4ᵒC until used.
Identification of bacterial isolates
After the purification, bacterial isolates were identified based on microscopic, morphological and biochemical charachers following Bergey,s Manual of systematic bacteriology (Bergey, 1984; Iqbal et al., 2015; Yunus et al., 2016).
Screening of bacteria isolates for anti-fungal activity
All bacterial isolates were streaked on Nutrient Agar at 37 ᵒC for 24 hr. Target fungi were seeded in Sabouraud Dextrose Agar. Discs of NA with growth of bacteria isolates were cut by cork borer (1cm diameter) and transferred to the surface of seeded target microorganism plates under aseptic conditions. These plates were kept for 1hr in the refrigerator to facilitate diffusion then incubated at 28 ᵒC for 3 days. Antifungal activity was recorded in term of inhibition zone of target fungal growth around the agar disc of bacterial isolates (Tepe et al., 2004).
Results
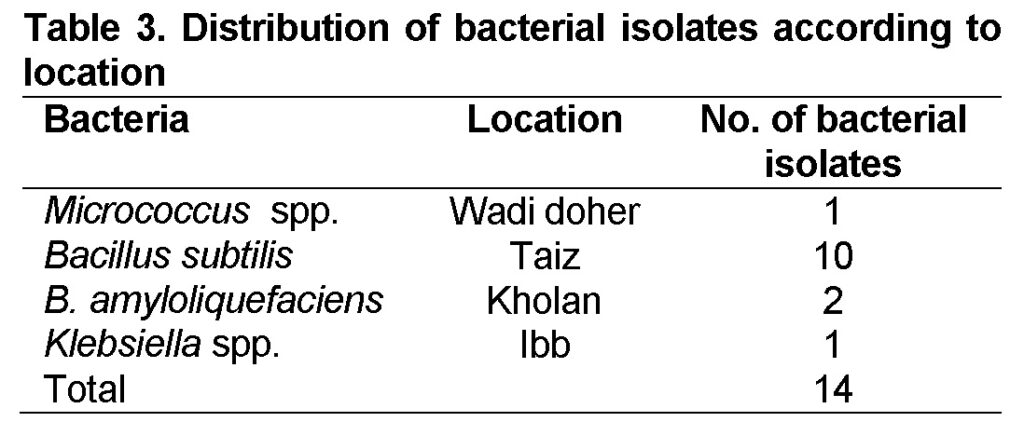
The results showed that a total of 14 bacteria isolates were collected from 10 soil samples from different localities in Yemen (Table 3). These isolates belong to four different species Klebsila spp., Bacillus subtilis, B. amyloliquefaciens and Micrococcus spp.
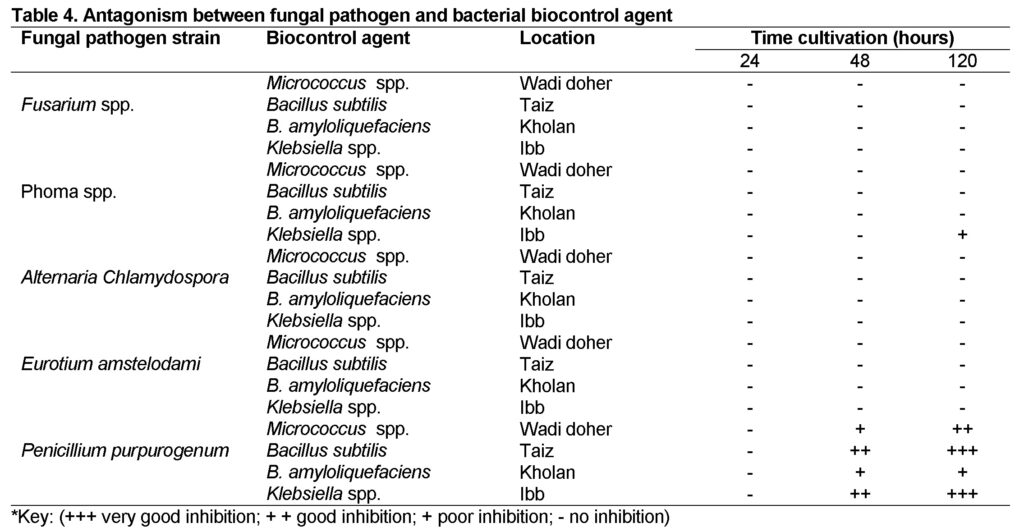
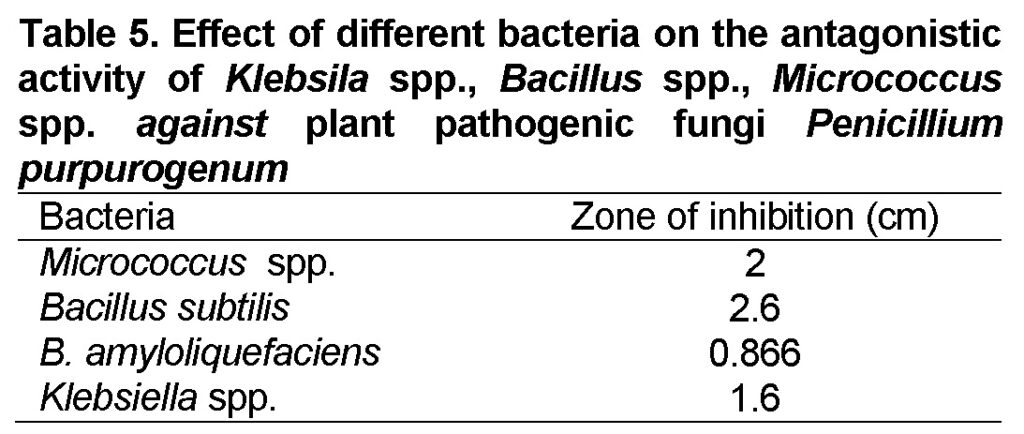
The isolated bacterial genera were tested against fungal species for their antifungal activity. The highest inhibition was shown against fungus Penicillium purpurogenum (Table 4). The average diameter of inhibition zone (cm) of bacterial isolates againstplant pathogenic fungi Penicillium purpurogenum was recorded as Klebsila spp. 2cm, Bacillus subtilis 2.6cm, B. amyloliquefaciens0.866cm and Micrococcus spp. 1.6cm (Table 5).
Discussion
In the present study, to maximize the chance for isolating bacteria suitable as rich sources of antifungal bioactive metabolites as well as for possible expansion of naturally occurring antifungal compounds for pathogenic fungi, we isolated bacteria from Yemen (Alhodida, Almahwite, Amran, Bni Al-harith, Bni Matar, Dhahban, Ibb, Kholan, Taiz, Wadi Dhahr) soil samples. A total of 14 isolates belonging to 4 different species of bacteria with inhibitory activity against selected fungi were isolated from soil samples and identified biochemically. They were recognized in the genera Bacillus (B. subtilis, and B. amyloliquefaciens), Klebsila sp, and Micrococcus sp (an unidentified species). Inhibitory bacteria with antifungal activity against some selected fungi were evaluated. All these species exhibited considerable antifungal activity in various grades, indicate that diversity of population is an extremely important factor determining the potential for antagonistic activity of bacteria toward various microorganisms. The antagonism between microbial strains can be explained in a number of ways: the most common are synthesis of metabolites, competition and direct parasitism, but other mechanisms are involved, for example induced resistance sometimes linked with reduction of pathogen enzyme activity.
As reported by other researchers, the genera Bacillus, Micrococcus spp.and Klebsila spp.had been well-thought-out as chief bacterial genera capable of showing antifungal activities (Ongena and Jacques, 2007). In the present study, the genus Bacillus was the most important antagonistic bacterial group as its isolates showed 0.866cm and 2.6cm zone on inhibition while the Micrococcus spp. showed 1.6 cm inhibition zone and the genus Klebsila spp. shown about 2 cm inhibition zone.
Within Bacillus species, B. subtilis as the most important species and in some extents other species such as B. amyloliquefaciens and B. valismortis are reported to produce a wide range of structurally related antimicrobial compounds and they are usually isolated from the soil as the main natural habitat (Stein, 2005; Arrebola et al., 2010). The strong inhibitory activity of Bacillus species isolated in the current research might aspect of manufacture of antifungal peptides by Bacillus strains (Reddy et al., 2009).
With respect to the considerable tolerance of B. subtilis to environmental stresses and their facile production by current fermentation technology, bacterial isolates identified in this study with a diverse range of antifungal activities can be known as prospective resources of novel naturally occurring antifungal agents for controlling pathogenic fungi in medicine and agriculture.
Clark (1996) stated that 2 or 3 antibiotics on average derived from microbes come in the marketplace every year. Similarly, bio-control products depending on bacteria have nowadays been economically established for the control of fungal diseases (Sharma et al., 2009). It has been shown that manufacture of tremendously widespread collection of antifungal composites by bacteria and their potential for use in bio control programs is fully dependent on limitations i.e; position of taxonomy and characteristics of physiology (i.e. species, varieties and growth cycle) conditions of geography, composition of soil, etc. Therefore, isolation of a huge amount of microbes commencing various geographical positions might raise the accidence of discovery of novel antifungal compounds.
Conclusion and Recommendations
Despite a lot of progress is made in the knowledge of the modes of action of biological control agents (BCAs), practical application usually fails to control disease in the fields. One of the reasons illustrating this failure is that the bio-control product is used the same manner as a chemical product. Being biological these products have to be used according to their ecological requirements. Another approach consists of introduction of plant defense reactions. This can be achieved by application of natural substances obtained from microorganisms, plants, or algae. A third approach comprises of selecting cultural practices that might reduce the occurrence or severity of diseases. Future studies to identify antifungal metabolites of antagonistic bacteria isolated here, to determine their mechanisms of action on fungal cells and their evaluation as effective fungal bio control agents in the field are recommended.
Acknowledgement
Authors are very grateful to Professor Abdelrahman A. Humaid, Microbiology Section, Biology Department – Faculty of Science, Sana’a University, Yemen for technical assistance.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Arrebola, E., Jacobs, R., Korsten, L., 2010. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol., 108(2):386-395.
Bergey, S.A., 1984. Bergey, Manual of Determinative Bacteriology, 9th edition, Williams & Wilkins., Philadelphia.
Clark, A.M., 1996. Natural products as a resource for new drugs. Pharm. Res., 1996;13(8):1133-1141.
Gallo, A., Giuberti, G., Frisvad, J.C., Bertuzzi, T., Nielsen, K.F., 2015. Review on mycotoxin issues in ruminants: occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins, 7(8): 3057-3111.
Ghisalberti, E.L., 2000. Bioactive metabolites from soilborne fungi: natural fungicides and biocontrol agents, Volume 21, Part 2: Bioactive Natural Products (Part B) Studies in Natural Products Chemistry. In: Atta-ur-Rahman, editor. Elsevier Science Publication, pp. 181-250.
Iqbal, M.N., Anjum, A.A., Ali, M.A., Hussain, F., ALI, S., Muhammad, A., Irfan, M., Ahmad, A., Irfan, M. and Shabbir, A., 2015. Assessment of microbial load of un-pasteurized fruit juices and in vitro antibacterial potential of honey against bacterial isolates. Open Microbiol. J., 9: 26-32. DOI: 10.2174/1874285820150601E001.
Lucas, G.B., Campbell, C.L., Lucas, L.T., 1992. Diseases caused by soil–borne fungi: In introduction to plant diseases: Identification and management. Second edition. Edited by G. B. Lucas, C.L. Campbell and L.T. Lucas. Van No strand Reinhold, New York, pp.162-191.
Ongena, M., Jacques, P., 2007. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol., 16:115-125.
Ranjbariyan, A.R., Shams-Ghahfarokhi, M., Kalantari, S., Razzaghi-Abyaneh, M., 2011. Molecular identification of antagonistic bacteria from Tehran soils and evaluation of their inhibitory activities toward pathogenic fungi. Iran J. Microbiol., 3(3): 140-146.
Reddy, B.P., Reddy, M.S., Kumar, K.V.K., 2009. Characterization of antifungal metabolites of Pseudomonas fluorescens and their effect on mycelia growth of Magnaporthe grisea and Rhizoctonia solani . Int. J. Pharm. Tech. Res., 1(4):1490-1493.
Samson, A.R., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., 2000. The Netherlands: CBS-Utrecht; Introduction to Food and Airborne Fungi. Sixth edn.
Sharma, R.R., Singh, D., Singh, R., 2009. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control., 50(3): 205-221.
Stein, T., 2005. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol. Microbiol., 56(4): 845-857.
Tepe, B., Donmez, E., Unlu, M., Candan, F., Daferera, D., Unlu, V., 2004. Antimicrobial and antioxidative activities of the essential oils and methanol extracts of Salvia cryptantha and Salvia multicaulis. J. Food Chem., 84(4): 519-525.
Yunus, F.N., Kanwal, F., Rashid, F., Ashraf, A., Iqbal, M.N., Xiao, S., 2016. A Comparative Study on Isolation and Identification of Bacillus thuringiensis from Different Localities of Gujranwala City. PSM Biol. Res.,01(1): 34-38.
Zivkovic, S., Stojanovic, S., Ivanovic, Z., Gavrilovic, V., Popovic, T., Balaz, J., 2010. Screening of antagonistic activity of microorganism against Colletotrichum aculatum and Colletotrichum gloeosporoides. Arch. Biol. Sci. Belgrade., 62(3): 611-623.