Evaluation of Changes in Liver Enzymes in Broiler Chicks (Gallous domesticus)
1Department of Zoology, Govt. Post Graduate Islamia College (W) Cooper Road, Lahore54000, Pakistan.
2The School of Life Sciences, Fujian Agriculture and Forestry University, Fuzhou 350002, China
3Pakistan Science Mission (PSM), Noor Kot 51770, Pakistan.
4Department of Zoology, Lahore College for Women University, Lahore, 54000, Pakistan.
5Department of Anatomy and Histology, University of Veterinary and Animal Sciences, Lahore 54000, Pakistan
6Department of Zoology, University of Poonch, Rawalakot 12350, Azad Kashmir, Pakistan.
7Department of Zoology, PMAS Arid Agriculture University, Rawalpindi 46000, Pakistan.
*Corresponding author:
Asfa Ashraf;Email:
sundausnaeem@yahoo.comViews 3754
Abstract
The study was conducted to evaluate the effect of lead administered as lead acetate at different dosage levels mixed with feed in Broiler Chicks. Eighty four chicks (one month old) were purchased from Tolinton market, Lahore. The chicks were allowed to acclimatize to laboratory condition in well- ventilated cages. They were divided into four groups having three replicas (7 chicks each). One of these was kept as control A (un-medicated). While the other Groups such as B, C, and D were given dose of lead acetate in a single dose at the pace of 80, 160 and 240 mg/kg of body mass mixed in feed for 20 days one after the other. Control diet Group “A” received basal diet without any supplementation. Blood samples were collected after slaughtering on 1st, 5th, 10th, 15th and 20th day of dietary treatment from each group. The serum was isolated according to the procedure reported in Manual of basic Techniques for healthy laboratory. Various serological parameters of liver function enzymes test (LFT) such as Alanine aminotransferase (ALT) and Aspartate aminotransferase (AST) were determined. Liver function tests are usually recognized as the reliable indicator of liver metabolism. A significant increase in ALT and AST levels (p< 0.05) in liver of various treated groups was recorded after applying statistical ANOVA test. Increased AST activity is related with the necrosis of the liver cells that leads to an escape of these enzymes into the blood. The level of ALT is generally increased in situations where there is damage to the liver cell membrane. Lead acetate alters the serum AST and ALT levels by affecting the liver. The results revealed that lead toxicity caused hepatotoxicity in broiler chicks.
Keywords
Broiler Chicks, serological parameters, liver function enzymes test, Alanine aminotransferase, Aspartate aminotransferase.
Citation
Khanam, F., Iqbal, M.N., Ashraf, A., Yunus, F.N., Alam, S., Muhammad, A., Xiao, S., Toor, S., Mumtaz, H., 2016. Evaluation of Changes in Liver Enzymes in Broiler Chicks (Gallous domesticus). PSM Vet. Res., 01(1): 26-31.
Introduction
Lead is a toxicant for virtually all organs of the body and has significant debilitating effects on the nervous, renal, hepatic and hematopoietic systems. Absorption of the Lead occurs in the blood plasma where it elaborates with the extracellular juice, crosses membranes such as the blood intellect barriers, the placenta, and circulates in spongy tissues and bones. Once absorbed, it is distributed particularly to the liver, kidneys, heart and gonads, as well as to the immune system. The storage tissue of organisms in which up to 90% of the total lead accumulates is the bone tissue (Verity, 1990). Erdogan et al., (2005) tested the lead one of the environmental pollutants that that causes many types of physiological and biochemical disorders in animals. Most of the metabolic processes occur in liver, which plays very important role in the regulation of lead metabolism (Sivaprasad et al., 2004). Youssef et al., (1996) investigated the consequence of toxic substance namely lead acetate at different doses going on the immune response in broiler chicks.
Foods of animal’s origin do not usually have excessive lead concentrations. The tissues in which lead is found in highest concentration are liver, kidneys and bone but its concentrations are usually much lower in milk than blood levels (Donia, 2008). Level of Lead is different in different types of animals (buffalo, cattle and others); but in meat muscles it is more than the acceptable amount even in the chicken meat its concentration is very high (Iwegbue et al., 2008). Birds can get exposure to lead by a number of ways via atmosphere, stream, foodstuff, lead batteries, paints, insect killer and petrol. Earlier researchers have established trends of decreasing net brain weight with increasing amount of lead in birds (Park et al., 2001).
Meat is a very cheap source of nutrition and micro-elements. Kind and degree of the feeding animal are the main factors which effects the chemical composition of meat. (Baykovet al., 1996; Hamasalim and Mohammed, 2013). These metals enter the food chain and results in bioaccumulation and biomagnification in the food chain (Demirezen and Uruc, 2006). Food chain translocated lead to organisms, its toxic effects depend on various factors such as its chemistry, the way of administration and the rate and extent administered to animals (Baht and Moy, 1997).
Hepatotoxicity is defined as the dysfunction and damage of liver with an abundance of drugs (Navarro and Senior, 2006). The chemicals that cause liver injury are called hepatotoxins or hepatotoxicants. Certain drugs may cause liver injury when used even within the therapeutic ranges (Willett et al., 2004; Papay et al., 2009).
The aim of our study was to evaluate the toxicity of lead acetate on the liver enzymes of chicks.
Materials and Methods
Experimental Birds
The experiment was conducted in Zoology department of Post graduate Islamia college (W) Cooper road, Lahore. The experimental birds were 1 month old clinically healthy eighty four chicks (Gallus domesticus). These chicks (n=84) were purchased from the local Tolinton market Lahore, Pakistan.
Acclimatization
The chicks were allowed to acclimatize to laboratory condition in well- ventilated cages for 5 days. They had access to feed and water all the time. These experimental animals were kept under standard conditions of temperature (37 °C) and humidity (40-50%). Care of the animals was taken as per guidelines of European Communities Council Directive of 24 November 1986 (86/609/EEC) and the study was approved by Institutional Animal Ethics Committee.
Sex ratio
After attaining the age of 2 months the male and female chicks were separated and placed in a cage in a ratio of 1:2. Surplus males or females were ruled out in all the experimental groups.
Toxicant
Toxicant used was Lead (II) acetate 3 hydrate, purchased from BDH Laboratory Supplies. This toxicant was mixed in feed with varying amount.
Body weight
Body weight was determined by an electric balance (SHIMADZU BX-3000 Japan). The chicks were weighted on very first day of experiment to have initial body weight and then on weekly bases to calculate the body weight changes till the termination of the experiment. The weight changing was calculated by previous body weight from new body weight.
Grouping of the broiler chicks
Total of the 84 chicks were divide into four groups. Each group contains 21 animals. These groups were further subdivided into 3 replicas each containing the seven chicks. One of these groups (A) was kept as a Control group. Other three (B, C, D) groups were served as Experimental groups.
Treatments
Experimental diets with different levels of lead acetate (B, C and D) were given to the chicks according to their body weights, mixed with commercial feed. Our pattern of selected doses was based on previous experiment performed by Suleman et al., (2011).
Group A
This group was kept as a control as they received normal feed and tap water.
Group B
The chicks were given lead acetate dose of 80mg/kg of body weight mixed in feed and tap water.
Group C
The chicks were given lead acetate dose of 160mg/kg of body weight mixed in feed and tap water.
Group D
The chicks were given lead acetate dose of 240mg/kg of body weight mixed in feed and water.
Blood collection
The blood samples were collected on weekly basis by slaughtering the animals. 10ml blood was collected in test tubes for serological studies (LFT).
Serum separation
The serum was isolated according to the procedure reported in Manual of basic Techniques for healthy laboratory.
Storage of serum
The labeled sampling ependoff tubes containing serum was kept in the freezer compartment of the refrigerator at -8 oC to -10 oC or below for at least one month.
Serological studies
Serum was analyzed to evaluate the changes in the level of enzymes using Crescent diagnostic kits.
Biochemical Procedures
Biochemical tests for ALT and ALP determination were performed following procedures used by Muhammad et al. (2013) and Toor et al. (2016).
Statistical analysis
Statistical analysis was performed through general linear models. Univeriate ANOVA was applied for the parameters (Scheffe, 1959) at 95% confidence interval. The data was expressed as mean ±SD abbreviated as standard deviation.
Results
Aspartate Aminotransferase (AST)
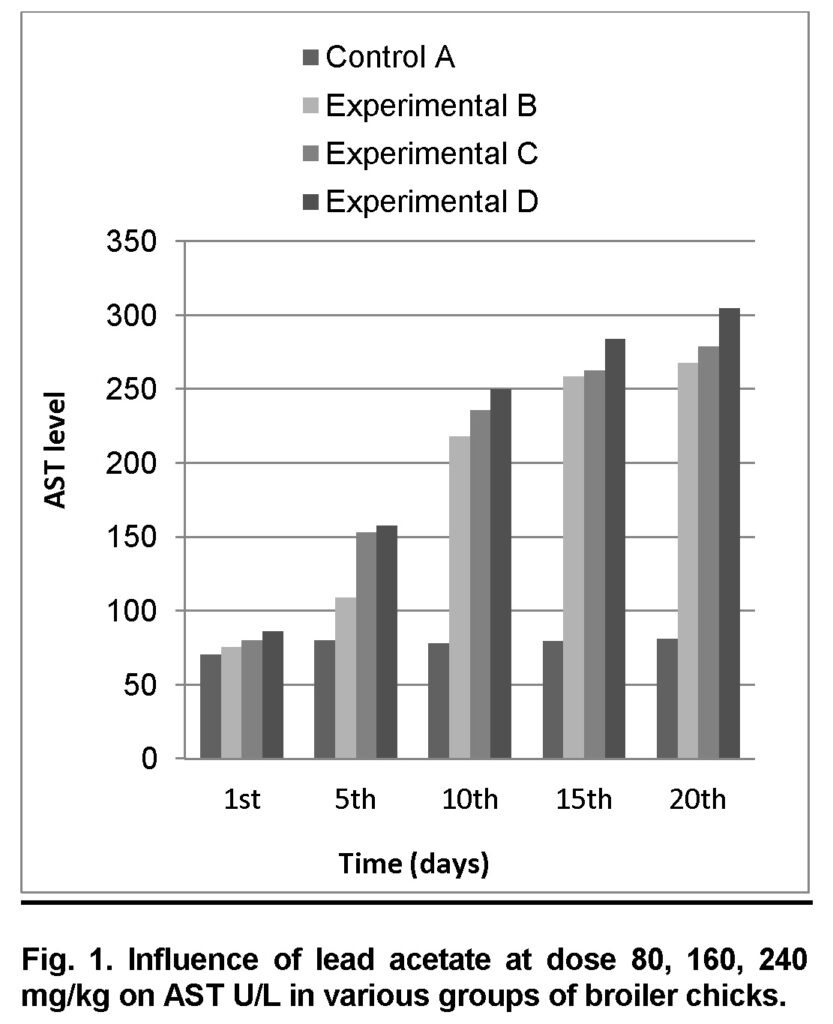
Influence of lead acetate at dose of 80mg/kg in group “B” was analyzed. The group A was served “Control”. The values of AST level in experimental group B on 1st, 5th, 10th, 15th and 20th days were 76 ± 0.96, 109 ± 0.42, 218 ± 2.32, 259 ± 1.14 and 268 ± 0.8 U/L respectively (Table 1). AST level is increased significantly in group “B” as compared to control at the end of 20th day of the experiment (Figure 1). The value of F was 7.465 showed highly significant results. The value of significance is 0.026, is less than 0.05.
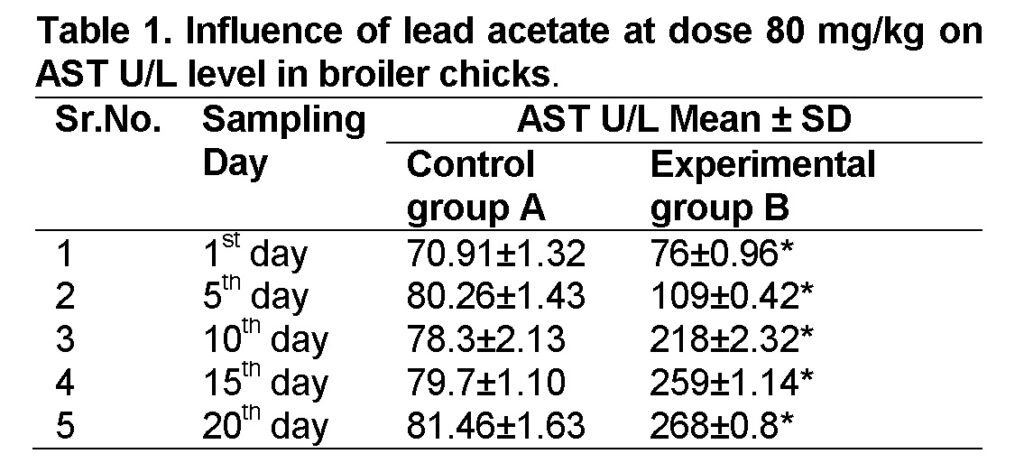
Influence of lead acetate at dose of 160mg/kg in group “C” was observed. The values of AST in experimental group C on 1st, 5th, 10th, 15th and 20th days were 80.2 ± 1.23, 153.5 ± 1.43, 236 ± 1.73, 263 ± 1.32 and 279 ± 1.30 respectively (Table 2). AST level increased significantly in group “C” as compared to control at the end of 20th day of the experiment (Figure 1). The value of F was 10.996 showed highly significant results. The value of significance was 0.011, is less than 0.05.
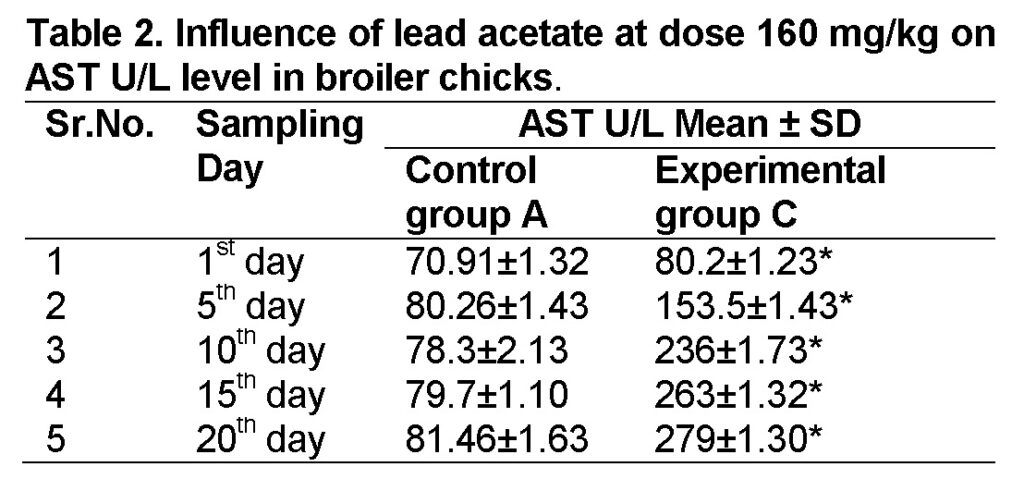
Influence of lead acetate at dose of 240mg/kg in group “D” was analyzed. The values of AST in experimental group D on 1st, 5th, 10th, 15th and 20th days were 86.5 ± 1.41, 158 ± 1.40, 250 ± 1.32, 284 ± 1.30 and 305 ± 0.93 respectively (Table 3). AST level increased significantly in group “D” as compared to control at the end of 20th day of the experiment (Figure 1). The value of F was 11.328 showed highly significant results. The value of significance was 0.010, is less than 0.05.
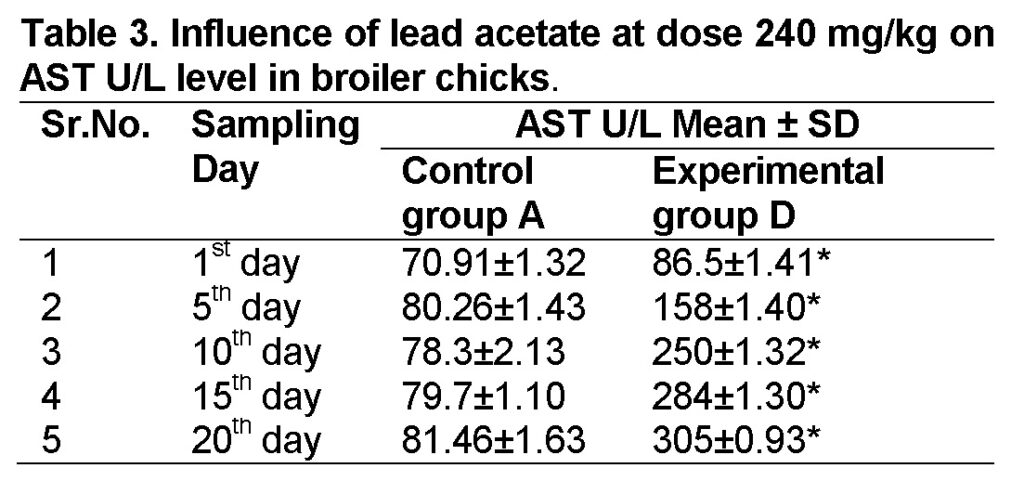
Alanine Aminotransferase (ALT)
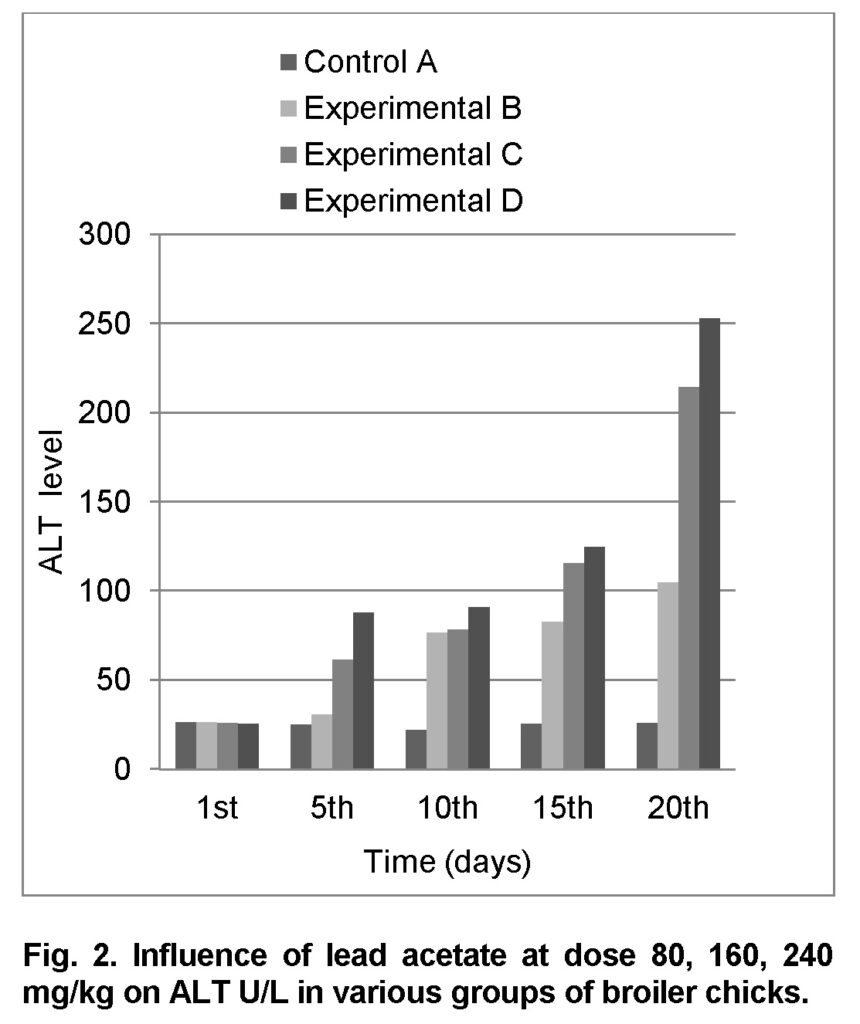
Influence of lead acetate at dose of 80mg/kg in group “B” was observed. The group A was served as “Control”. The levels of ALT in experimental group B on 1st, 5th, 10th, 15th and 20th days were 26.4 ± 1.32, 31 ± 3.97, 77 ± 2.80, 83 ± 2.28 and 105 ± 2.00 respectively (Table 4). ALT level increased significantly in group “B” as compared to control at the end of 20th day of the experiment (Figure 2). The value of F was 6.545 showed highly significant results. The value of significance was 0.034, is less than 0.05.
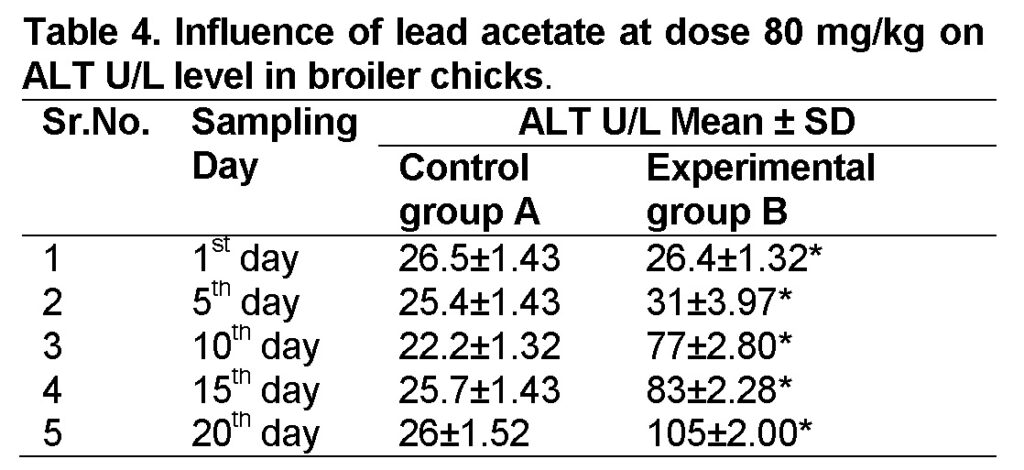
Influence of lead acetate at dose of 160mg/kg in group “C” was observed. The values of ALT in experimental group C on 1st, 5th, 10th, 15th and 20th days were 26.3 ± 1.33, 61.5 ± 2.35, 78.5 ± 2.30, 116 ± 2.34 and 214.5 ± 1.09 respectively (Table 5). ALT level increased significantly in group “C” as compared to control at the end of 20th day of the experiment (Figure 2). The value of F is 5.306 showed highly significant results. The value of significance was 0.05.
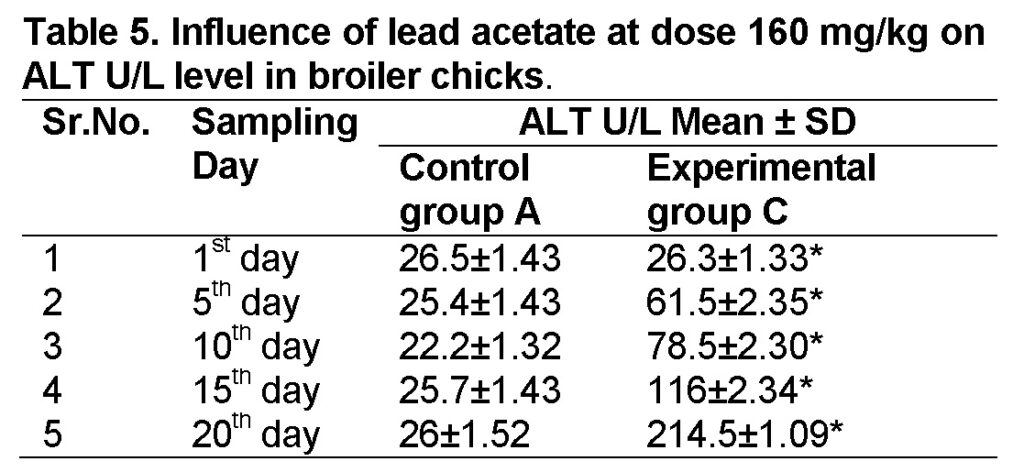
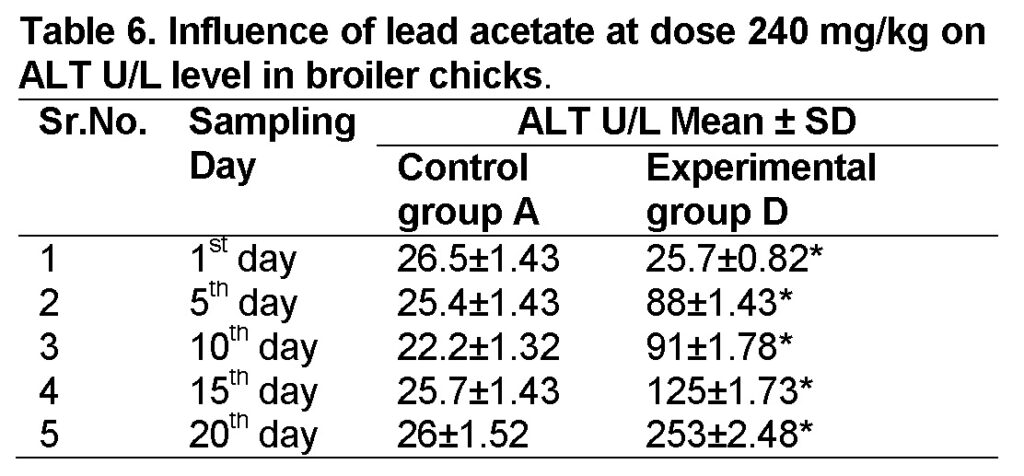
Influence of lead acetate at dose of 240mg/kg in group “D” was observed. The values of ALT in experimental group D on 1st, 5th, 10th, 15th and 20th days were 25.7 ± 0.82, 88 ± 1.43, 91 ± 1.78, 125 ± 1.73 and 253 ± 2.48 respectively (Table 6). The increase in ALT level is significant in group “D” as compared to control at the end of 20th day of the experiment. The value of F was 5.876 showed highly significant results. The value of significance was 0.042, is less than 0.05.
Discussion
In current study, the lead acetate toxicity on the liver functioning enzymes (AST & ALT) was investigated in broiler chicks (Gallus domesticus). The liver is largely affected by lead toxicity owing to its storage in the liver after lead exposure. The elevation in level of enzymes might be due to higher cell membrane permeability or damage of hepatocytes due to lead acetate (Tatjanaet al., 2003).
Increased AST activity is related with the necrosis of the liver cells leads to an escape of these enzymes into the blood. In acute hepatitis the level of these enzymes are increased sometimes 10 or even 100 folds. The increased effects the plasma ALT (normally 4 – 13) even more than plasma AST (normally S-17 IU/l), and these changes may proceed the onset of clinical Jaundice as mentioned by Walter and Israel, (1987). In acute muscle injury and muscle inflammatory conditions there is an increase in AST.
According to Benjamin, (1986) muscle stress and muscular damage increase the AST levels and higher than those of normal conditions. The amount of AST is directly related to hepatocytes damage (Khan, 2001).
During acute inflammatory conditions of the liver, ALT higher evaluations are directly related to hepatocellular disorderness. In the presence of liver disease, ALT is raised due to leakage of this enzyme into the blood stream. Bishop, (2000) and Khan, (2001) showed increase in ALT concentration directly related to the amount of damage to hepatocytes.
Raphael, (1976) reported that hepatic necrosis is not necessary for the evaluation of ALT, since an alternation in cell membrane permeability an all that is required to leak this cytoplasmic enzymes into the blood. According to him the second reason of normal to mediate evaluation of ALT is associated with passive congestion of the liver and fatty metamorphosis. So it might be the reason of increase level of ALT as congestion was observed in present work.
In present study there was a marked increase in AST and ALT seen in treatment of 20th day having 240 mg/kg of lead acetate were 305 ± 0.93 and 253 ± 2.48 respectively. Whereas mild significant increase was observed on 1st, 5th, 10th 15th days of the treatment on AST were 86.5 ± 1.41, 158 ± 1.40, 250 ± 1.32, 284 ± 1.30 and on ALT levels were 25.7 ± 0.82, 88 ± 1.43, 91 ± 1.78, 125 ± 1.73 respectively.
Ibrahim et al., 2011 reported that the influence of lead acetate ingestion in male albino rats have high stimulation of the AST and ALT activity. In addition, Abdou et al., (2007) also reported the hepatotoxic effect of lead. This indicated that effect of lead acetate on the activity of transaminases is dose independent. The high liver microsomal membrane fluidity, free radical generation, and alteration in the liver tissue histogram are responsible for high plasma ALT and AST activity (Seddik et al., 2010).
Al-Wabel et al, (2007) has demonstrated the same results in rat treated with lead acetate showing the increased level of ALT. Similar results have also been documented by Ashmawy et al, (2005), who illustrated that lead acetate has a toxic effect on liver increasing the level of ALT. In a previous study of effect of lead acetate administered orally at various dosage levels in Broiler chicks, a significant increase in AST in chicks was reported by Suleman et al ., (2011) as same is the case in our study.
Conclusion
From the present study, it has been shown that lead acetate changes the serum AST and ALT levels in broiler chickens by affecting the liver. The toxic signs were much more obvious when high doses were used whereas there was no prominent toxicity present when low dose of lead acetate were used. However, the presence of lead in liver causes hepatotoxicity.
Acknowledgement
The authors are grateful to Ms. Huma Mumtaz, Department of Zoology, Govt.Post Graduate Islamia College (W) Cooper Road, Lahore for providing materials to carry out this research.
Conflict of Interest
The authors declare that this article content has no conflict of interest.
References
Abdou, Z.A., Attia, M., Raafat, M.A., 2007. Protective effect of citric acid and thiol compounds against cadmium and lead toxicity in experimental animals. J. Biol. Chem. Environ. Sci., 2(2), 481-497.
Al-Wabel, N.A., Mousa, H.M., Omer, O.H., Abdel-Salam, A.M., 2007. Biological evaluation of symbiotic fermented milk against lead ace contamination in rats. J. Food, Agric. Environ., 5 (3, 4): 169-172.
Ashmawy, I.M., El-Nahas, A.F., Salama, O.M., 2005. Protective effect of volatile oil, alcoholic and aqueous extracts of Origanummajorana on lead acetate toxicity in mice. Basic. Clin. Pharmacol. Toxicol., 97(4): 238-43.
Baht, R.V., Moy, G.G., 1997. Monitoring and assessment of dietary exposure to chemical contaminants. Geneva. WHO., 32: 132-149.
Baykov, B.D., Stoyanov, M.P., Gugova, M.L., 1996. Cadmium and lead bioaccumulation in male chickens for high food concentration.Toxicol. Environ. Chem., 54: 155-159.
Benjamin, M.M., 1986. Outline of veterinary clinical pathology, 3rd Ed. The lowa state university press. Ames. Lowa. USA. pp. 3114-3125.
Bishop, M.L., 2000. Clinical chemistry. Lippincot William and Wilkin A Wolter Kluwer Company, Philadelphia. pp. 256-237.
Burger, J., Gochfeld, M., 1995. Growth and behavioral effects of early postnatal chromium and manganese exposure in herring gull (Larusargentatus) chicks. Pharmacol. Biochem. Behav., 50: 607-612.
Demirezen, D., Urue, K., 2006. Comparative study of trace elements in certain fish, meat and product. Meat. Sci., 74(2): 255-260.
Donia, A.M.A., 2008. Lead concentrations in different animal’s muscles and consumable organs at specific localities in Cairo. Glob. Vet., 2(5): 280-284.
Erdogan, Z., Erdogan, S., Aksu, T., Baytok, E., 2005. The effect of dietary lead exposure and ascorbic acid on performance, lipid peroxidation status and biochemical parameters of broilers. Turk. J. Vet. Anim. Sci., 29: 1053-1059.
Hamasalim, J.H., Mohammed, N.H., 2013. Determination of heavy metals in exposed corned beef and chicken luncheon that sold in Sulaymaniah markets. Afr. J. Food Sci., 7(7): 178-182.
Ibrahim, M.N., Eweis, A.E., El-Beltagi, S.H., Abdel-Mobdy, E.Y., 2011. The effect of lead acetate toxicity on experimental male albino rat. Biol. Trace. Elem. Res., 144: 1120-1132.
Iwegbue, G.M.A., Nwajei, G.E., Iyoha, E.H., 2008. Heavy metal residues of chicken, meat and gizzard and turkey meat consumed in southern Nigeria. Bulg. J. Vet. Med., 11(4): 275-280.
Khan, S.A., 2001.Manual of veterinary clinical pathology. Maktaba-e-Danishwaran, Lahore, Pakistan. pp. 457-496.
Muhammad, A., Farooq, M.U., Iqbal, M.N., Ali, S., Ahmad, A., Irfan, M., 2013. Prevalence of diabetes mellitus type II in patients with hepatitis C and association with other risk factors. Punjab Univ. J. Zool., 28 (2): 69-75.
Navarro, V.J., Senior, J.R., 2006. Drug-related hepatotoxicity. N. Engl. J. Med., 354: 731-739.
Papay, J.I., Clines, D., Rafi, R., Yuen, N., Britt, S.D., 2009. Drug-induced liver injury following positive drug rechallenge. Regul. Toxicol. Pharmacol., 54: 84-90.
Park, J., Porter, N., Franklin, E.W., College, M., 2001. The effects of lead acetate on the neural development of chick embryos. Cell. Biochem. Embr., 3: 315-317.
Raphael, S.S., 1976. In: Lunch’s medical laboratory technology. W. B. Saunders Company, Philadelphia. London. pp. 177-178.
Scheffe, H., 1959. The analysis of variance. John Willey and Sons, New York. Pp. 245-257.
Seddik, L., Bah, T.M., Aoues, A., Brnderdour, M., Silmani, M., 2010. Dried leaf extract protects against lead induced neurotoxicity in wistar rats. Eur. J. Sci. Res., 42(1): 139-151.
Sivaprasad, R., Nagaraj, M., Varalakshmi, P., 2004. Combined efficacies of lipoic acid and 2, 3- dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J. Nutr. Biochem., 15: 18-23.
Suleman, M., Khan, A.A., Hussain, Z., Zia, A.M., Rashid, F.R.S., Iqbal, A., Ishaq, R., 2011. Effect of lead acetate administered orally at different dosage levels in broiler chicks. Afr. J. Environ. Sci. Technol., 5(12): 1017-1026.
Tatjana, J., Gordana, K., Dusica, P., Ivana, S., 2003. Effects of captopril on membrane associated enzymes in lead induced hepatotoxicity in rats. Acta. Fac. Med. Naiss., 20: 183-188.
Toor, S., Toor, S., Ashraf, A., Alam, S., Anwaar, S., Saddiqa, A., Ali, S., Muhammad, A., Toor, S., Akhter, S., 2016. Prevalence of Liver disorders in Islamabad City. PSM Biol. Res., 01(1): 31-33.
Verity, A.M., 1990.Comparative observations on inorganic and organic lead neurotoxicity. Environ. Health Perspect., 89: 43-48.
Walter, J.B., Israel, M.S., 1987. In General pathology Churchil living stone. Edinburg London Melbourne and New York. pp. 604-627.
Willett, K.L., Roth, R.A., Walker, L., 2004. Workshop overview: Hepatotoxicity assessment for botanical dietary supplements. Toxicol. Sci., 79: 4-9.
Youssef, S.A.H., El- Sanousi, A.A., Afifi, N.A., El-Brawy, A.M.A., 1996. Effect of subclinical lead toxicity on the immune response of chickens to newcastle disease virus vaccine. Res. Vet. Sci., 60(1): 13-16.